 Found 109 hits of Enzyme Inhibition Constant Data
Found 109 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240973
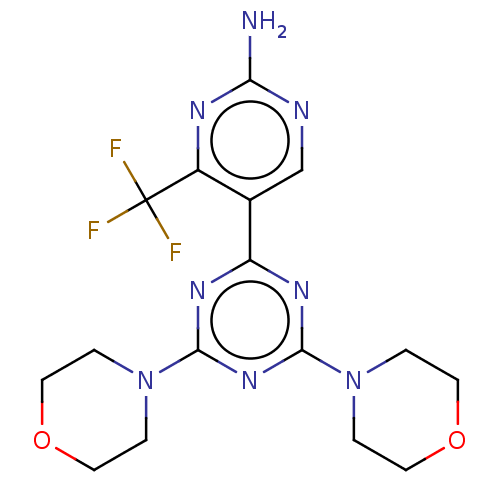
(CHEMBL4102855)Show SMILES Nc1ncc(-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C16H19F3N8O2/c17-16(18,19)11-10(9-21-13(20)22-11)12-23-14(26-1-5-28-6-2-26)25-15(24-12)27-3-7-29-8-4-27/h9H,1-8H2,(H2,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240989
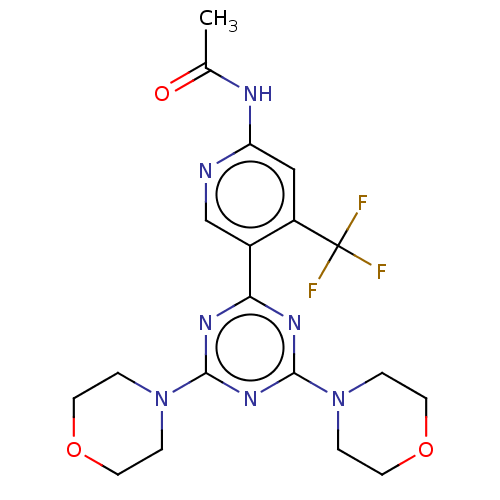
(CHEMBL4081904)Show SMILES CC(=O)Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C19H22F3N7O3/c1-12(30)24-15-10-14(19(20,21)22)13(11-23-15)16-25-17(28-2-6-31-7-3-28)27-18(26-16)29-4-8-32-9-5-29/h10-11H,2-9H2,1H3,(H,23,24,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240991
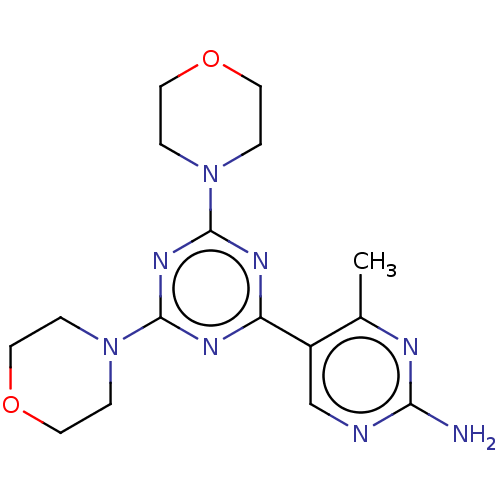
(CHEMBL4092165)Show InChI InChI=1S/C16H22N8O2/c1-11-12(10-18-14(17)19-11)13-20-15(23-2-6-25-7-3-23)22-16(21-13)24-4-8-26-9-5-24/h10H,2-9H2,1H3,(H2,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240992
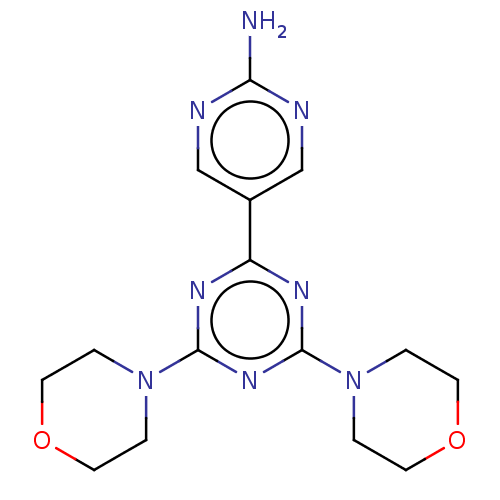
(CHEMBL4097282)Show InChI InChI=1S/C15H20N8O2/c16-13-17-9-11(10-18-13)12-19-14(22-1-5-24-6-2-22)21-15(20-12)23-3-7-25-8-4-23/h9-10H,1-8H2,(H2,16,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240977
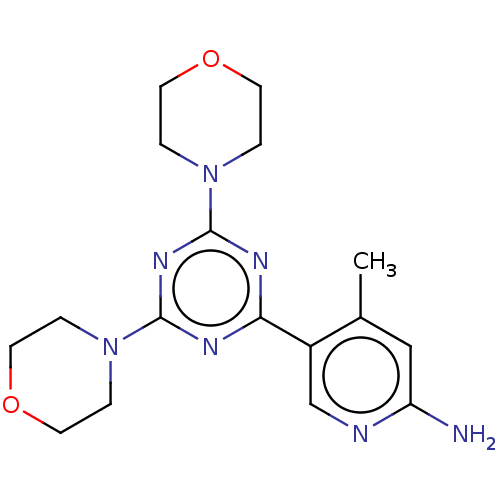
(CHEMBL4071150)Show InChI InChI=1S/C17H23N7O2/c1-12-10-14(18)19-11-13(12)15-20-16(23-2-6-25-7-3-23)22-17(21-15)24-4-8-26-9-5-24/h10-11H,2-9H2,1H3,(H2,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240992

(CHEMBL4097282)Show InChI InChI=1S/C15H20N8O2/c16-13-17-9-11(10-18-13)12-19-14(22-1-5-24-6-2-22)21-15(20-12)23-3-7-25-8-4-23/h9-10H,1-8H2,(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240984
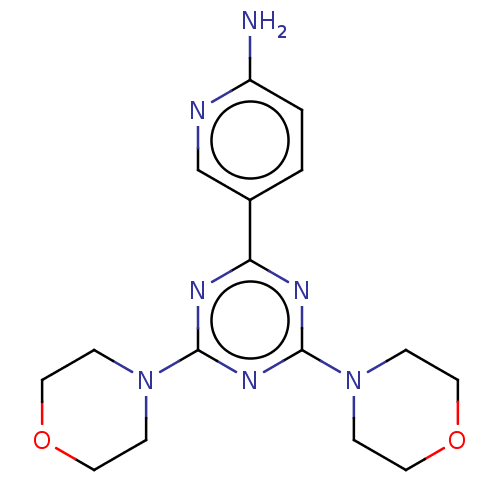
(CHEMBL4068426)Show InChI InChI=1S/C16H21N7O2/c17-13-2-1-12(11-18-13)14-19-15(22-3-7-24-8-4-22)21-16(20-14)23-5-9-25-10-6-23/h1-2,11H,3-10H2,(H2,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240984

(CHEMBL4068426)Show InChI InChI=1S/C16H21N7O2/c17-13-2-1-12(11-18-13)14-19-15(22-3-7-24-8-4-22)21-16(20-14)23-5-9-25-10-6-23/h1-2,11H,3-10H2,(H2,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240983
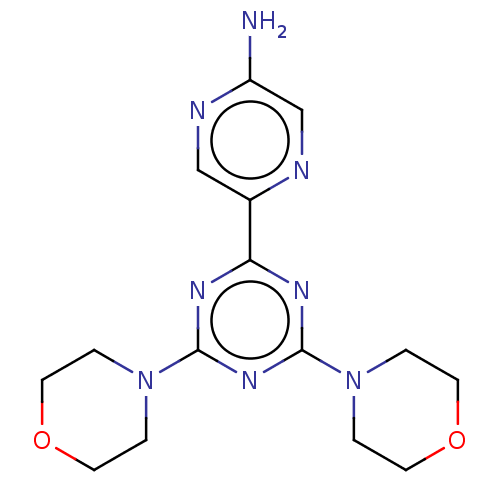
(CHEMBL4079258)Show InChI InChI=1S/C15H20N8O2/c16-12-10-17-11(9-18-12)13-19-14(22-1-5-24-6-2-22)21-15(20-13)23-3-7-25-8-4-23/h9-10H,1-8H2,(H2,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240973

(CHEMBL4102855)Show SMILES Nc1ncc(-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C16H19F3N8O2/c17-16(18,19)11-10(9-21-13(20)22-11)12-23-14(26-1-5-28-6-2-26)25-15(24-12)27-3-7-29-8-4-27/h9H,1-8H2,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240991

(CHEMBL4092165)Show InChI InChI=1S/C16H22N8O2/c1-11-12(10-18-14(17)19-11)13-20-15(23-2-6-25-7-3-23)22-16(21-13)24-4-8-26-9-5-24/h10H,2-9H2,1H3,(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240976

(CHEMBL4098203)Show InChI InChI=1S/C17H23N7O2/c1-12-10-13(11-19-14(12)18)15-20-16(23-2-6-25-7-3-23)22-17(21-15)24-4-8-26-9-5-24/h10-11H,2-9H2,1H3,(H2,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 241 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240995
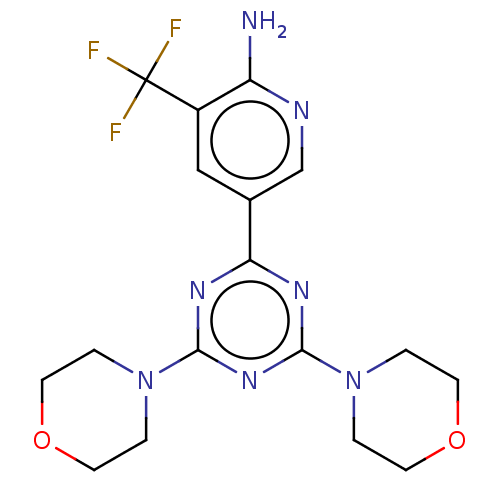
(CHEMBL4071370)Show SMILES Nc1ncc(cc1C(F)(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-11(10-22-13(12)21)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 284 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240985
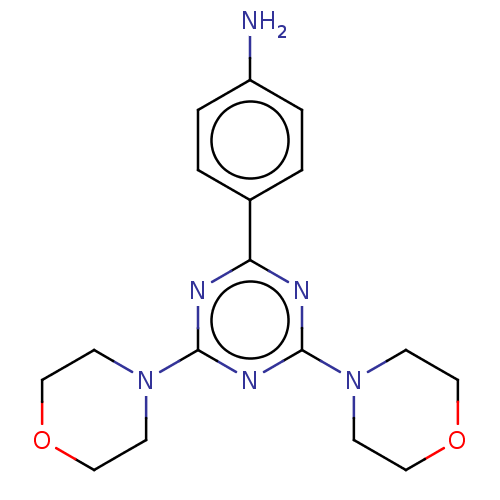
(CHEMBL4089567)Show InChI InChI=1S/C17H22N6O2/c18-14-3-1-13(2-4-14)15-19-16(22-5-9-24-10-6-22)21-17(20-15)23-7-11-25-12-8-23/h1-4H,5-12,18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 396 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240977

(CHEMBL4071150)Show InChI InChI=1S/C17H23N7O2/c1-12-10-14(18)19-11-13(12)15-20-16(23-2-6-25-7-3-23)22-17(21-15)24-4-8-26-9-5-24/h10-11H,2-9H2,1H3,(H2,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 609 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240993
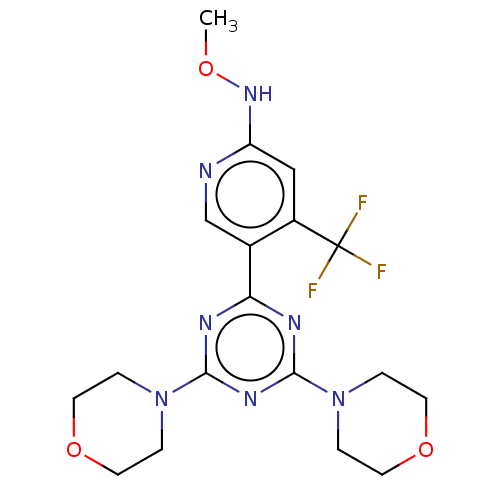
(CHEMBL4074098)Show SMILES CONc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H22F3N7O3/c1-29-26-14-10-13(18(19,20)21)12(11-22-14)15-23-16(27-2-6-30-7-3-27)25-17(24-15)28-4-8-31-9-5-28/h10-11H,2-9H2,1H3,(H,22,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 689 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240983

(CHEMBL4079258)Show InChI InChI=1S/C15H20N8O2/c16-12-10-17-11(9-18-12)13-19-14(22-1-5-24-6-2-22)21-15(20-13)23-3-7-25-8-4-23/h9-10H,1-8H2,(H2,16,18) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 911 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240976

(CHEMBL4098203)Show InChI InChI=1S/C17H23N7O2/c1-12-10-13(11-19-14(12)18)15-20-16(23-2-6-25-7-3-23)22-17(21-15)24-4-8-26-9-5-24/h10-11H,2-9H2,1H3,(H2,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240989

(CHEMBL4081904)Show SMILES CC(=O)Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C19H22F3N7O3/c1-12(30)24-15-10-14(19(20,21)22)13(11-23-15)16-25-17(28-2-6-31-7-3-28)27-18(26-16)29-4-8-32-9-5-29/h10-11H,2-9H2,1H3,(H,23,24,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240985

(CHEMBL4089567)Show InChI InChI=1S/C17H22N6O2/c18-14-3-1-13(2-4-14)15-19-16(22-5-9-24-10-6-22)21-17(20-15)23-7-11-25-12-8-23/h1-4H,5-12,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240994

(CHEMBL4099904)Show SMILES Oc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H19F3N6O3/c18-17(19,20)12-9-13(27)21-10-11(12)14-22-15(25-1-5-28-6-2-25)24-16(23-14)26-3-7-29-8-4-26/h9-10H,1-8H2,(H,21,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240993

(CHEMBL4074098)Show SMILES CONc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H22F3N7O3/c1-29-26-14-10-13(18(19,20)21)12(11-22-14)15-23-16(27-2-6-30-7-3-27)25-17(24-15)28-4-8-31-9-5-28/h10-11H,2-9H2,1H3,(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240988
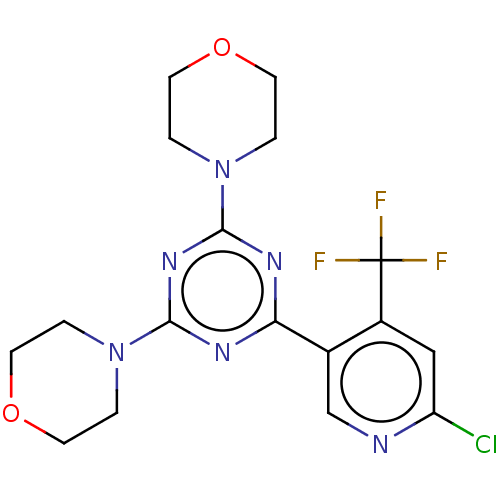
(CHEMBL4100862)Show SMILES FC(F)(F)c1cc(Cl)ncc1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C17H18ClF3N6O2/c18-13-9-12(17(19,20)21)11(10-22-13)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240988

(CHEMBL4100862)Show SMILES FC(F)(F)c1cc(Cl)ncc1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C17H18ClF3N6O2/c18-13-9-12(17(19,20)21)11(10-22-13)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240987
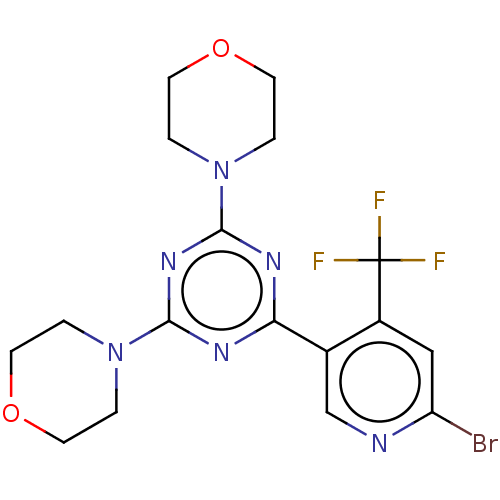
(CHEMBL4062723)Show SMILES FC(F)(F)c1cc(Br)ncc1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C17H18BrF3N6O2/c18-13-9-12(17(19,20)21)11(10-22-13)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Displacement of [3H]baclofen from gamma-aminobutyric-acid B receptor |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240995

(CHEMBL4071370)Show SMILES Nc1ncc(cc1C(F)(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-11(10-22-13(12)21)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380363
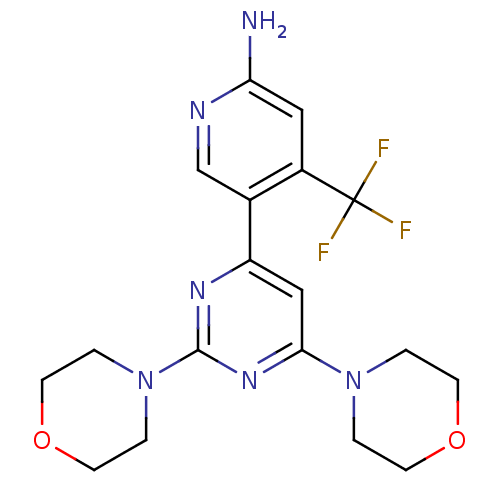
(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Displacement of [3H]baclofen from gamma-aminobutyric-acid B receptor |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Displacement of [3H]baclofen from gamma-aminobutyric-acid B receptor |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human A2058 cells assessed as reduction in phosphorylation of S6 at ser235/236 residues after 1 hr by in-cell Western method |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240973

(CHEMBL4102855)Show SMILES Nc1ncc(-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C16H19F3N8O2/c17-16(18,19)11-10(9-21-13(20)22-11)12-23-14(26-1-5-28-6-2-26)25-15(24-12)27-3-7-29-8-4-27/h9H,1-8H2,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human A2058 cells assessed as reduction in phosphorylation of S6 at ser235/236 residues after 1 hr by in-cell Western method |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240984

(CHEMBL4068426)Show InChI InChI=1S/C16H21N7O2/c17-13-2-1-12(11-18-13)14-19-15(22-3-7-24-8-4-22)21-16(20-14)23-5-9-25-10-6-23/h1-2,11H,3-10H2,(H2,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 415 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human A2058 cells assessed as reduction in phosphorylation of S6 at ser235/236 residues after 1 hr by in-cell Western method |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240992

(CHEMBL4097282)Show InChI InChI=1S/C15H20N8O2/c16-13-17-9-11(10-18-13)12-19-14(22-1-5-24-6-2-22)21-15(20-12)23-3-7-25-8-4-23/h9-10H,1-8H2,(H2,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 415 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human A2058 cells assessed as reduction in phosphorylation of S6 at ser235/236 residues after 1 hr by in-cell Western method |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 451 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Displacement of [3H]baclofen from gamma-aminobutyric-acid B receptor |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 607 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Displacement of [3H]baclofen from gamma-aminobutyric-acid B receptor |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 661 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Displacement of [3H]baclofen from gamma-aminobutyric-acid B receptor |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240989

(CHEMBL4081904)Show SMILES CC(=O)Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C19H22F3N7O3/c1-12(30)24-15-10-14(19(20,21)22)13(11-23-15)16-25-17(28-2-6-31-7-3-28)27-18(26-16)29-4-8-32-9-5-29/h10-11H,2-9H2,1H3,(H,23,24,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 729 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human A2058 cells assessed as reduction in phosphorylation of S6 at ser235/236 residues after 1 hr by in-cell Western method |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 778 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Displacement of [3H]baclofen from gamma-aminobutyric-acid B receptor |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240983

(CHEMBL4079258)Show InChI InChI=1S/C15H20N8O2/c16-12-10-17-11(9-18-12)13-19-14(22-1-5-24-6-2-22)21-15(20-13)23-3-7-25-8-4-23/h9-10H,1-8H2,(H2,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 817 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human A2058 cells assessed as reduction in phosphorylation of S6 at ser235/236 residues after 1 hr by in-cell Western method |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240991

(CHEMBL4092165)Show InChI InChI=1S/C16H22N8O2/c1-11-12(10-18-14(17)19-11)13-20-15(23-2-6-25-7-3-23)22-16(21-13)24-4-8-26-9-5-24/h10H,2-9H2,1H3,(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 831 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human A2058 cells assessed as reduction in phosphorylation of S6 at ser235/236 residues after 1 hr by in-cell Western method |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240977

(CHEMBL4071150)Show InChI InChI=1S/C17H23N7O2/c1-12-10-14(18)19-11-13(12)15-20-16(23-2-6-25-7-3-23)22-17(21-15)24-4-8-26-9-5-24/h10-11H,2-9H2,1H3,(H2,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 939 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human A2058 cells assessed as reduction in phosphorylation of S6 at ser235/236 residues after 1 hr by in-cell Western method |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240976

(CHEMBL4098203)Show InChI InChI=1S/C17H23N7O2/c1-12-10-13(11-19-14(12)18)15-20-16(23-2-6-25-7-3-23)22-17(21-15)24-4-8-26-9-5-24/h10-11H,2-9H2,1H3,(H2,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human A2058 cells assessed as reduction in phosphorylation of S6 at ser235/236 residues after 1 hr by in-cell Western method |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240985

(CHEMBL4089567)Show InChI InChI=1S/C17H22N6O2/c18-14-3-1-13(2-4-14)15-19-16(22-5-9-24-10-6-22)21-17(20-15)23-7-11-25-12-8-23/h1-4H,5-12,18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human A2058 cells assessed as reduction in phosphorylation of S6 at ser235/236 residues after 1 hr by in-cell Western method |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240995

(CHEMBL4071370)Show SMILES Nc1ncc(cc1C(F)(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-11(10-22-13(12)21)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human A2058 cells assessed as reduction in phosphorylation of S6 at ser235/236 residues after 1 hr by in-cell Western method |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 (unknown origin) |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240993

(CHEMBL4074098)Show SMILES CONc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H22F3N7O3/c1-29-26-14-10-13(18(19,20)21)12(11-22-14)15-23-16(27-2-6-30-7-3-27)25-17(24-15)28-4-8-31-9-5-28/h10-11H,2-9H2,1H3,(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human A2058 cells assessed as reduction in phosphorylation of S6 at ser235/236 residues after 1 hr by in-cell Western method |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of DNAPK (unknown origin) |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 (unknown origin) |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of DNAPK (unknown origin) |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240988

(CHEMBL4100862)Show SMILES FC(F)(F)c1cc(Cl)ncc1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C17H18ClF3N6O2/c18-13-9-12(17(19,20)21)11(10-22-13)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human A2058 cells assessed as reduction in phosphorylation of S6 at ser235/236 residues after 1 hr by in-cell Western method |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240994

(CHEMBL4099904)Show SMILES Oc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H19F3N6O3/c18-17(19,20)12-9-13(27)21-10-11(12)14-22-15(25-1-5-28-6-2-25)24-16(23-14)26-3-7-29-8-4-26/h9-10H,1-8H2,(H,21,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human A2058 cells assessed as reduction in phosphorylation of S6 at ser235/236 residues after 1 hr by in-cell Western method |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
In vitro renin inhibition was measured at pH 7.4 by using purified human kidney renin assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to DNAPK (unknown origin) by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase beta
(Homo sapiens (Human)) | BDBM50240973

(CHEMBL4102855)Show SMILES Nc1ncc(-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C16H19F3N8O2/c17-16(18,19)11-10(9-21-13(20)22-11)12-23-14(26-1-5-28-6-2-26)25-15(24-12)27-3-7-29-8-4-27/h9H,1-8H2,(H2,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI4KCbeta (M1 to M828 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kalpha (R108 to N1068 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25028
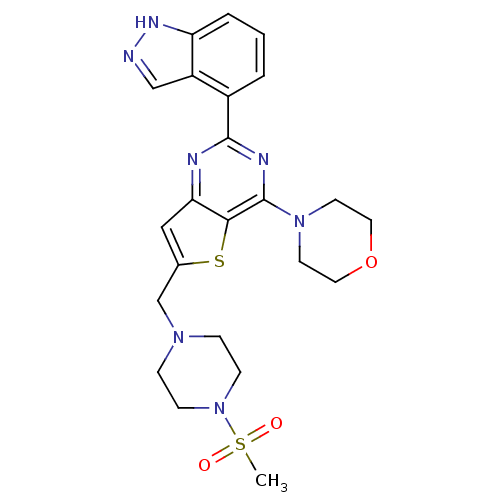
(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kalpha (R108 to N1068 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50240973

(CHEMBL4102855)Show SMILES Nc1ncc(-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C16H19F3N8O2/c17-16(18,19)11-10(9-21-13(20)22-11)12-23-14(26-1-5-28-6-2-26)25-15(24-12)27-3-7-29-8-4-27/h9H,1-8H2,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to rat striatal dopamine receptor D2 was determined in vitro |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to DNAPK (unknown origin) by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase beta
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI4KCbeta (M1 to M828 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human mTOR (L1382 to W2549 residues) expressed in mammalian expression system at 10 uM |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50308135
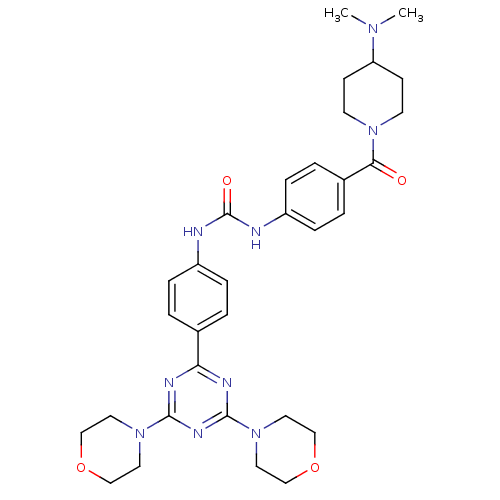
(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3KC2beta (M1 to L1634 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3KC2beta (M1 to L1634 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50240973

(CHEMBL4102855)Show SMILES Nc1ncc(-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C16H19F3N8O2/c17-16(18,19)11-10(9-21-13(20)22-11)12-23-14(26-1-5-28-6-2-26)25-15(24-12)27-3-7-29-8-4-27/h9H,1-8H2,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to rat striatal dopamine receptor D2 was determined in vitro |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50240973

(CHEMBL4102855)Show SMILES Nc1ncc(-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C16H19F3N8O2/c17-16(18,19)11-10(9-21-13(20)22-11)12-23-14(26-1-5-28-6-2-26)25-15(24-12)27-3-7-29-8-4-27/h9H,1-8H2,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308135

(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human mTOR (L1382 to W2549 residues) expressed in mammalian expression system at 10 uM |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human mTOR (L1382 to W2549 residues) expressed in mammalian expression system at 10 uM |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kalpha (R108 to N1068 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50240973

(CHEMBL4102855)Show SMILES Nc1ncc(-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C16H19F3N8O2/c17-16(18,19)11-10(9-21-13(20)22-11)12-23-14(26-1-5-28-6-2-26)25-15(24-12)27-3-7-29-8-4-27/h9H,1-8H2,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
In vitro renin inhibition was measured at pH 7.4 by using purified human kidney renin assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50240973

(CHEMBL4102855)Show SMILES Nc1ncc(-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C16H19F3N8O2/c17-16(18,19)11-10(9-21-13(20)22-11)12-23-14(26-1-5-28-6-2-26)25-15(24-12)27-3-7-29-8-4-27/h9H,1-8H2,(H2,20,21,22) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
In vitro renin inhibition was measured at pH 7.4 by using purified human kidney renin assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50308135

(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to DNAPK (unknown origin) by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase beta
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI4KCbeta (M1 to M828 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human VPS34 (S282 to H879 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kbeta (P118 to S1070 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50308135

(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human mTOR (L1382 to W2549 residues) expressed in mammalian expression system at 10 uM |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50240973

(CHEMBL4102855)Show SMILES Nc1ncc(-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C16H19F3N8O2/c17-16(18,19)11-10(9-21-13(20)22-11)12-23-14(26-1-5-28-6-2-26)25-15(24-12)27-3-7-29-8-4-27/h9H,1-8H2,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
In vitro renin inhibition was measured at pH 7.4 by using purified human kidney renin assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
In vitro renin inhibition was measured at pH 7.4 by using purified human kidney renin assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50240973

(CHEMBL4102855)Show SMILES Nc1ncc(-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C16H19F3N8O2/c17-16(18,19)11-10(9-21-13(20)22-11)12-23-14(26-1-5-28-6-2-26)25-15(24-12)27-3-7-29-8-4-27/h9H,1-8H2,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kalpha (R108 to N1068 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50308135

(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kbeta (P118 to S1070 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308135

(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kalpha (R108 to N1068 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50308135

(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human VPS34 (S282 to H879 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase beta
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI4KCbeta (M1 to M828 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase beta
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI4KCbeta (M1 to M828 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kalpha (R108 to N1068 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kbeta (P118 to S1070 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3KC2beta (M1 to L1634 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kbeta (P118 to S1070 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50240973

(CHEMBL4102855)Show SMILES Nc1ncc(-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C16H19F3N8O2/c17-16(18,19)11-10(9-21-13(20)22-11)12-23-14(26-1-5-28-6-2-26)25-15(24-12)27-3-7-29-8-4-27/h9H,1-8H2,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
In vitro renin inhibition was measured at pH 7.4 by using purified human kidney renin assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50308135

(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50380363

(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50240975

(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kbeta (P118 to S1070 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase beta
(Homo sapiens (Human)) | BDBM50308135

(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI4KCbeta (M1 to M828 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50358204

(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human VPS34 (S282 to H879 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data