 Found 21 hits of Enzyme Inhibition Constant Data
Found 21 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Rattus norvegicus) | BDBM50021153
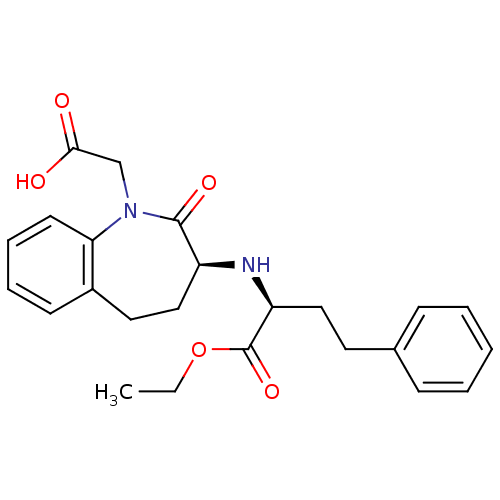
(1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C24H28N2O5/c1-2-31-24(30)20(14-12-17-8-4-3-5-9-17)25-19-15-13-18-10-6-7-11-21(18)26(23(19)29)16-22(27)28/h3-11,19-20,25H,2,12-16H2,1H3,(H,27,28)/t19-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021148
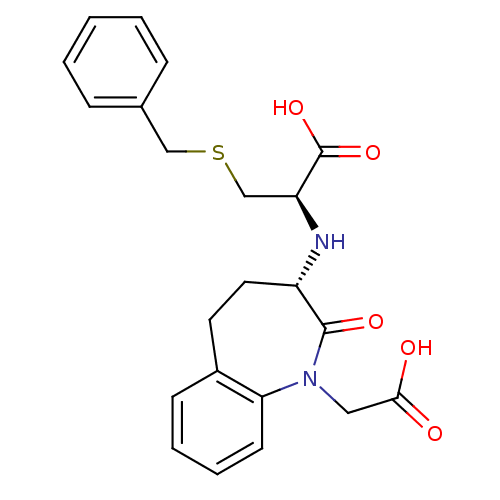
(3-Benzylsulfanyl-2-(1-carboxymethyl-2-oxo-2,3,4,5-...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](CSCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C22H24N2O5S/c25-20(26)12-24-19-9-5-4-8-16(19)10-11-17(21(24)27)23-18(22(28)29)14-30-13-15-6-2-1-3-7-15/h1-9,17-18,23H,10-14H2,(H,25,26)(H,28,29)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021151
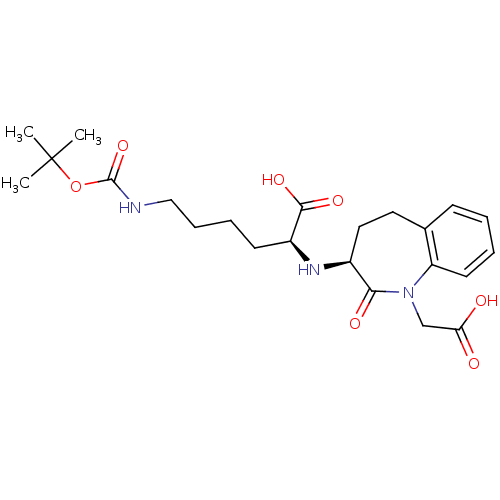
(6-tert-Butoxycarbonylamino-2-(1-carboxymethyl-2-ox...)Show SMILES CC(C)(C)OC(=O)NCCCC[C@H](N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O)C(O)=O Show InChI InChI=1S/C23H33N3O7/c1-23(2,3)33-22(32)24-13-7-6-9-17(21(30)31)25-16-12-11-15-8-4-5-10-18(15)26(20(16)29)14-19(27)28/h4-5,8,10,16-17,25H,6-7,9,11-14H2,1-3H3,(H,24,32)(H,27,28)(H,30,31)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021138

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](CSc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C21H22N2O5S/c24-19(25)12-23-18-9-5-4-6-14(18)10-11-16(20(23)26)22-17(21(27)28)13-29-15-7-2-1-3-8-15/h1-9,16-17,22H,10-13H2,(H,24,25)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50017129

((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...)Show SMILES CCOC(=O)[C@H](CCc1ccccc1)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C20H28N2O5/c1-3-27-20(26)16(12-11-15-8-5-4-6-9-15)21-14(2)18(23)22-13-7-10-17(22)19(24)25/h4-6,8-9,14,16-17,21H,3,7,10-13H2,1-2H3,(H,24,25)/t14-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021154

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES CCCC[C@H](N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O)C(O)=O Show InChI InChI=1S/C18H24N2O5/c1-2-3-7-14(18(24)25)19-13-10-9-12-6-4-5-8-15(12)20(17(13)23)11-16(21)22/h4-6,8,13-14,19H,2-3,7,9-11H2,1H3,(H,21,22)(H,24,25)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021149

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES CCC[C@H](N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O)C(O)=O Show InChI InChI=1S/C17H22N2O5/c1-2-5-13(17(23)24)18-12-9-8-11-6-3-4-7-14(11)19(16(12)22)10-15(20)21/h3-4,6-7,12-13,18H,2,5,8-10H2,1H3,(H,20,21)(H,23,24)/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021140

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES CSCC[C@H](N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O)C(O)=O Show InChI InChI=1S/C17H22N2O5S/c1-25-9-8-13(17(23)24)18-12-7-6-11-4-2-3-5-14(11)19(16(12)22)10-15(20)21/h2-5,12-13,18H,6-10H2,1H3,(H,20,21)(H,23,24)/t12-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021137

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](CSCCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C23H26N2O5S/c26-21(27)14-25-20-9-5-4-8-17(20)10-11-18(22(25)28)24-19(23(29)30)15-31-13-12-16-6-2-1-3-7-16/h1-9,18-19,24H,10-15H2,(H,26,27)(H,29,30)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021142

(6-Amino-2-(1-carboxymethyl-2-oxo-2,3,4,5-tetrahydr...)Show SMILES NCCCC[C@H](N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O)C(O)=O Show InChI InChI=1S/C18H25N3O5/c19-10-4-3-6-14(18(25)26)20-13-9-8-12-5-1-2-7-15(12)21(17(13)24)11-16(22)23/h1-2,5,7,13-14,20H,3-4,6,8-11,19H2,(H,22,23)(H,25,26)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021150

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](Cc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C21H22N2O5/c24-19(25)13-23-18-9-5-4-8-15(18)10-11-16(20(23)26)22-17(21(27)28)12-14-6-2-1-3-7-14/h1-9,16-17,22H,10-13H2,(H,24,25)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021152

(3-Benzyloxy-2-(1-carboxymethyl-2-oxo-2,3,4,5-tetra...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](COCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C22H24N2O6/c25-20(26)12-24-19-9-5-4-8-16(19)10-11-17(21(24)27)23-18(22(28)29)14-30-13-15-6-2-1-3-7-15/h1-9,17-18,23H,10-14H2,(H,25,26)(H,28,29)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021141

(6-Benzyloxycarbonylamino-2-(1-carboxymethyl-2-oxo-...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](CCCCNC(=O)OCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C26H31N3O7/c30-23(31)16-29-22-12-5-4-10-19(22)13-14-20(24(29)32)28-21(25(33)34)11-6-7-15-27-26(35)36-17-18-8-2-1-3-9-18/h1-5,8-10,12,20-21,28H,6-7,11,13-17H2,(H,27,35)(H,30,31)(H,33,34)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021144

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES OC(=O)CN1c2ccccc2CC[C@H](N[C@@H](Cc2c[nH]c3ccccc23)C(O)=O)C1=O Show InChI InChI=1S/C23H23N3O5/c27-21(28)13-26-20-8-4-1-5-14(20)9-10-18(22(26)29)25-19(23(30)31)11-15-12-24-17-7-3-2-6-16(15)17/h1-8,12,18-19,24-25H,9-11,13H2,(H,27,28)(H,30,31)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021143

(6-Benzyloxycarbonylamino-2-(1-carboxymethyl-2-oxo-...)Show SMILES CCOC(=O)[C@H](CCCCNC(=O)OCc1ccccc1)N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C28H35N3O7/c1-2-37-27(35)23(13-8-9-17-29-28(36)38-19-20-10-4-3-5-11-20)30-22-16-15-21-12-6-7-14-24(21)31(26(22)34)18-25(32)33/h3-7,10-12,14,22-23,30H,2,8-9,13,15-19H2,1H3,(H,29,36)(H,32,33)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021139

(6-Amino-2-(1-carboxymethyl-2-oxo-2,3,4,5-tetrahydr...)Show SMILES CCOC(=O)[C@H](CCCCN)N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C20H29N3O5/c1-2-28-20(27)16(8-5-6-12-21)22-15-11-10-14-7-3-4-9-17(14)23(19(15)26)13-18(24)25/h3-4,7,9,15-16,22H,2,5-6,8,10-13,21H2,1H3,(H,24,25)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021147

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES CC(C)C[C@H](N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O)C(O)=O Show InChI InChI=1S/C18H24N2O5/c1-11(2)9-14(18(24)25)19-13-8-7-12-5-3-4-6-15(12)20(17(13)23)10-16(21)22/h3-6,11,13-14,19H,7-10H2,1-2H3,(H,21,22)(H,24,25)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021136
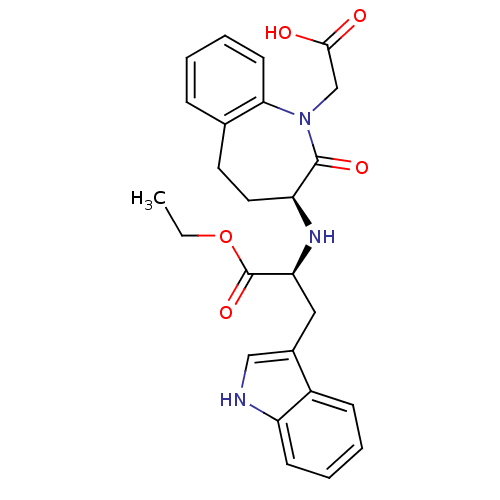
(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES CCOC(=O)[C@H](Cc1c[nH]c2ccccc12)N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C25H27N3O5/c1-2-33-25(32)21(13-17-14-26-19-9-5-4-8-18(17)19)27-20-12-11-16-7-3-6-10-22(16)28(24(20)31)15-23(29)30/h3-10,14,20-21,26-27H,2,11-13,15H2,1H3,(H,29,30)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021146

(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES COC(=O)[C@H](CC(C)C)N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O Show InChI InChI=1S/C19H26N2O5/c1-12(2)10-15(19(25)26-3)20-14-9-8-13-6-4-5-7-16(13)21(18(14)24)11-17(22)23/h4-7,12,14-15,20H,8-11H2,1-3H3,(H,22,23)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50021145
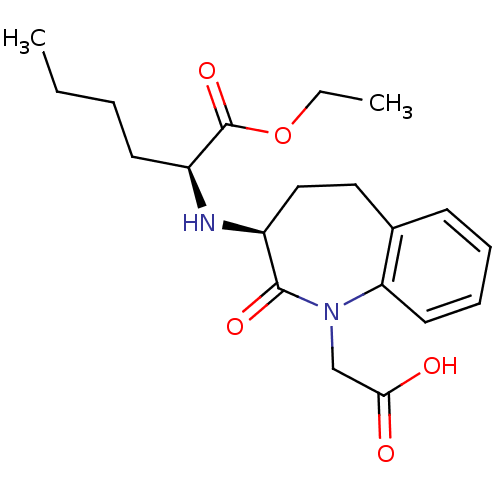
(2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...)Show SMILES CCCC[C@H](N[C@H]1CCc2ccccc2N(CC(O)=O)C1=O)C(=O)OCC Show InChI InChI=1S/C20H28N2O5/c1-3-5-9-16(20(26)27-4-2)21-15-12-11-14-8-6-7-10-17(14)22(19(15)25)13-18(23)24/h6-8,10,15-16,21H,3-5,9,11-13H2,1-2H3,(H,23,24)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin I converting enzyme |
J Med Chem 28: 1603-6 (1985)
BindingDB Entry DOI: 10.7270/Q2ZK5H7D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data