 Found 14 hits of Enzyme Inhibition Constant Data
Found 14 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V2 receptor
(Sus scrofa) | BDBM50452524

(CHEMBL2372291)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C51H74N12O10S2/c1-4-73-33-19-17-32(18-20-33)26-35-44(67)59-36(25-31-13-7-5-8-14-31)46(69)62-42(30(2)3)48(71)60-37(27-40(52)64)45(68)61-38(29-74-75-51(28-41(65)57-35)21-9-6-10-22-51)49(72)63-24-12-16-39(63)47(70)58-34(43(53)66)15-11-23-56-50(54)55/h5,7-8,13-14,17-20,30,34-39,42H,4,6,9-12,15-16,21-29H2,1-3H3,(H2,52,64)(H2,53,66)(H,57,65)(H,58,70)(H,59,67)(H,60,71)(H,61,68)(H,62,69)(H4,54,55,56)/t34-,35+,36-,37-,38?,39+,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... |
J Med Chem 31: 1487-9 (1988)
BindingDB Entry DOI: 10.7270/Q2S46QZN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50023754
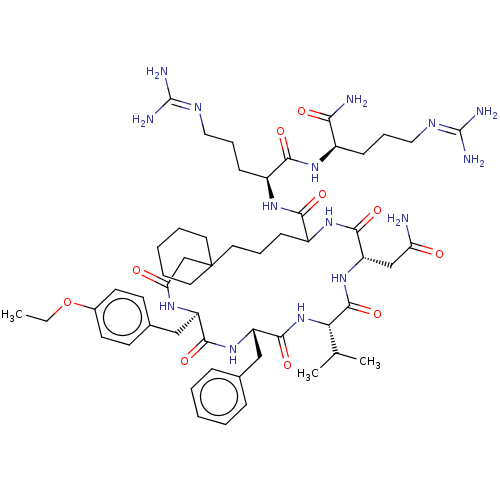
(13-Benzyl-19-carbamoylmethyl-10-(4-ethoxy-benzyl)-...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#6]-[#6]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C54H83N15O10/c1-4-79-35-21-19-34(20-22-35)29-39-48(75)67-40(28-33-14-7-5-8-15-33)50(77)69-44(32(2)3)51(78)68-41(30-42(55)70)49(76)66-37(16-11-25-54(31-43(71)63-39)23-9-6-10-24-54)47(74)65-38(18-13-27-62-53(59)60)46(73)64-36(45(56)72)17-12-26-61-52(57)58/h5,7-8,14-15,19-22,32,36-41,44H,4,6,9-13,16-18,23-31H2,1-3H3,(H2,55,70)(H2,56,72)(H,63,71)(H,64,73)(H,65,74)(H,66,76)(H,67,75)(H,68,78)(H,69,77)(H4,57,58,61)(H4,59,60,62)/t36-,37?,38+,39-,40+,41+,44+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... |
J Med Chem 31: 1487-9 (1988)
BindingDB Entry DOI: 10.7270/Q2S46QZN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50023750
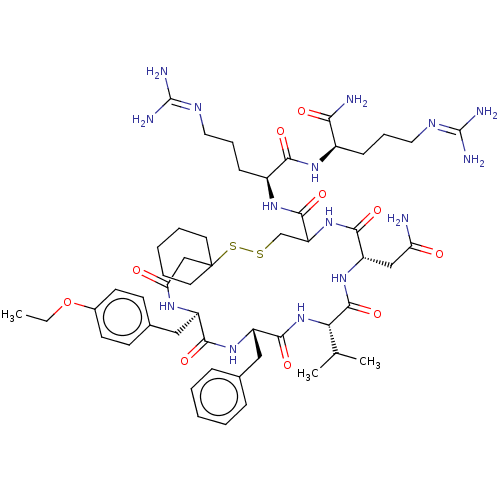
(1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C52H79N15O10S2/c1-4-77-33-19-17-32(18-20-33)26-36-45(72)64-37(25-31-13-7-5-8-14-31)47(74)67-42(30(2)3)49(76)65-38(27-40(53)68)46(73)66-39(29-78-79-52(28-41(69)61-36)21-9-6-10-22-52)48(75)63-35(16-12-24-60-51(57)58)44(71)62-34(43(54)70)15-11-23-59-50(55)56/h5,7-8,13-14,17-20,30,34-39,42H,4,6,9-12,15-16,21-29H2,1-3H3,(H2,53,68)(H2,54,70)(H,61,69)(H,62,71)(H,63,75)(H,64,72)(H,65,76)(H,66,73)(H,67,74)(H4,55,56,59)(H4,57,58,60)/t34-,35+,36-,37+,38+,39?,42+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... |
J Med Chem 31: 1487-9 (1988)
BindingDB Entry DOI: 10.7270/Q2S46QZN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50023750

(1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C52H79N15O10S2/c1-4-77-33-19-17-32(18-20-33)26-36-45(72)64-37(25-31-13-7-5-8-14-31)47(74)67-42(30(2)3)49(76)65-38(27-40(53)68)46(73)66-39(29-78-79-52(28-41(69)61-36)21-9-6-10-22-52)48(75)63-35(16-12-24-60-51(57)58)44(71)62-34(43(54)70)15-11-23-59-50(55)56/h5,7-8,13-14,17-20,30,34-39,42H,4,6,9-12,15-16,21-29H2,1-3H3,(H2,53,68)(H2,54,70)(H,61,69)(H,62,71)(H,63,75)(H,64,72)(H,65,76)(H,66,73)(H,67,74)(H4,55,56,59)(H4,57,58,60)/t34-,35+,36-,37+,38+,39?,42+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... |
J Med Chem 31: 1487-9 (1988)
BindingDB Entry DOI: 10.7270/Q2S46QZN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50023752
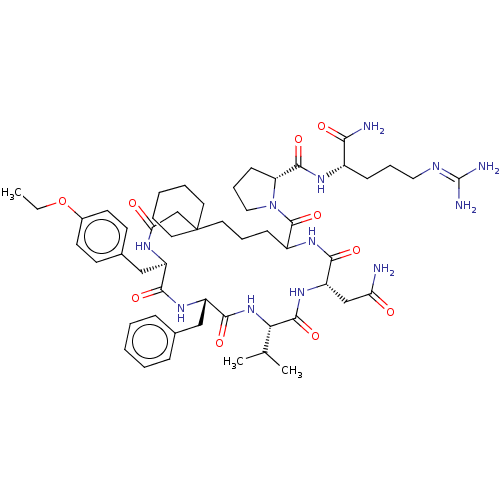
(1-[13-Benzyl-19-carbamoylmethyl-10-(4-ethoxy-benzy...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#6]-[#6]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C53H78N12O10/c1-4-75-35-21-19-34(20-22-35)29-38-46(69)62-39(28-33-14-7-5-8-15-33)48(71)64-44(32(2)3)50(73)63-40(30-42(54)66)47(70)61-37(16-11-25-53(31-43(67)59-38)23-9-6-10-24-53)51(74)65-27-13-18-41(65)49(72)60-36(45(55)68)17-12-26-58-52(56)57/h5,7-8,14-15,19-22,32,36-41,44H,4,6,9-13,16-18,23-31H2,1-3H3,(H2,54,66)(H2,55,68)(H,59,67)(H,60,72)(H,61,70)(H,62,69)(H,63,73)(H,64,71)(H4,56,57,58)/t36-,37?,38+,39-,40-,41+,44-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... |
J Med Chem 31: 1487-9 (1988)
BindingDB Entry DOI: 10.7270/Q2S46QZN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50452524

(CHEMBL2372291)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C51H74N12O10S2/c1-4-73-33-19-17-32(18-20-33)26-35-44(67)59-36(25-31-13-7-5-8-14-31)46(69)62-42(30(2)3)48(71)60-37(27-40(52)64)45(68)61-38(29-74-75-51(28-41(65)57-35)21-9-6-10-22-51)49(72)63-24-12-16-39(63)47(70)58-34(43(53)66)15-11-23-56-50(54)55/h5,7-8,13-14,17-20,30,34-39,42H,4,6,9-12,15-16,21-29H2,1-3H3,(H2,52,64)(H2,53,66)(H,57,65)(H,58,70)(H,59,67)(H,60,71)(H,61,68)(H,62,69)(H4,54,55,56)/t34-,35+,36-,37-,38?,39+,42-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... |
J Med Chem 31: 1487-9 (1988)
BindingDB Entry DOI: 10.7270/Q2S46QZN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50023751
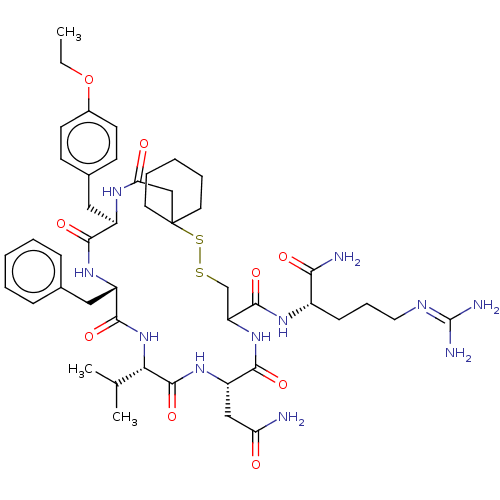
(19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C46H67N11O9S2/c1-4-66-30-17-15-29(16-18-30)23-32-40(61)54-33(22-28-12-7-5-8-13-28)42(63)57-38(27(2)3)44(65)55-34(24-36(47)58)41(62)56-35(43(64)53-31(39(48)60)14-11-21-51-45(49)50)26-67-68-46(25-37(59)52-32)19-9-6-10-20-46/h5,7-8,12-13,15-18,27,31-35,38H,4,6,9-11,14,19-26H2,1-3H3,(H2,47,58)(H2,48,60)(H,52,59)(H,53,64)(H,54,61)(H,55,65)(H,56,62)(H,57,63)(H4,49,50,51)/t31-,32+,33-,34-,35?,38-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... |
J Med Chem 31: 1487-9 (1988)
BindingDB Entry DOI: 10.7270/Q2S46QZN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50023751

(19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C46H67N11O9S2/c1-4-66-30-17-15-29(16-18-30)23-32-40(61)54-33(22-28-12-7-5-8-13-28)42(63)57-38(27(2)3)44(65)55-34(24-36(47)58)41(62)56-35(43(64)53-31(39(48)60)14-11-21-51-45(49)50)26-67-68-46(25-37(59)52-32)19-9-6-10-20-46/h5,7-8,12-13,15-18,27,31-35,38H,4,6,9-11,14,19-26H2,1-3H3,(H2,47,58)(H2,48,60)(H,52,59)(H,53,64)(H,54,61)(H,55,65)(H,56,62)(H,57,63)(H4,49,50,51)/t31-,32+,33-,34-,35?,38-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... |
J Med Chem 31: 1487-9 (1988)
BindingDB Entry DOI: 10.7270/Q2S46QZN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50023750

(1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C52H79N15O10S2/c1-4-77-33-19-17-32(18-20-33)26-36-45(72)64-37(25-31-13-7-5-8-14-31)47(74)67-42(30(2)3)49(76)65-38(27-40(53)68)46(73)66-39(29-78-79-52(28-41(69)61-36)21-9-6-10-22-52)48(75)63-35(16-12-24-60-51(57)58)44(71)62-34(43(54)70)15-11-23-59-50(55)56/h5,7-8,13-14,17-20,30,34-39,42H,4,6,9-12,15-16,21-29H2,1-3H3,(H2,53,68)(H2,54,70)(H,61,69)(H,62,71)(H,63,75)(H,64,72)(H,65,76)(H,66,73)(H,67,74)(H4,55,56,59)(H4,57,58,60)/t34-,35+,36-,37+,38+,39?,42+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... |
J Med Chem 31: 1487-9 (1988)
BindingDB Entry DOI: 10.7270/Q2S46QZN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50023752

(1-[13-Benzyl-19-carbamoylmethyl-10-(4-ethoxy-benzy...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#6]-[#6]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C53H78N12O10/c1-4-75-35-21-19-34(20-22-35)29-38-46(69)62-39(28-33-14-7-5-8-15-33)48(71)64-44(32(2)3)50(73)63-40(30-42(54)66)47(70)61-37(16-11-25-53(31-43(67)59-38)23-9-6-10-24-53)51(74)65-27-13-18-41(65)49(72)60-36(45(55)68)17-12-26-58-52(56)57/h5,7-8,14-15,19-22,32,36-41,44H,4,6,9-13,16-18,23-31H2,1-3H3,(H2,54,66)(H2,55,68)(H,59,67)(H,60,72)(H,61,70)(H,62,69)(H,63,73)(H,64,71)(H4,56,57,58)/t36-,37?,38+,39-,40-,41+,44-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... |
J Med Chem 31: 1487-9 (1988)
BindingDB Entry DOI: 10.7270/Q2S46QZN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50023752

(1-[13-Benzyl-19-carbamoylmethyl-10-(4-ethoxy-benzy...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#6]-[#6]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C53H78N12O10/c1-4-75-35-21-19-34(20-22-35)29-38-46(69)62-39(28-33-14-7-5-8-15-33)48(71)64-44(32(2)3)50(73)63-40(30-42(54)66)47(70)61-37(16-11-25-53(31-43(67)59-38)23-9-6-10-24-53)51(74)65-27-13-18-41(65)49(72)60-36(45(55)68)17-12-26-58-52(56)57/h5,7-8,14-15,19-22,32,36-41,44H,4,6,9-13,16-18,23-31H2,1-3H3,(H2,54,66)(H2,55,68)(H,59,67)(H,60,72)(H,61,70)(H,62,69)(H,63,73)(H,64,71)(H4,56,57,58)/t36-,37?,38+,39-,40-,41+,44-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonist activity was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in human |
J Med Chem 31: 1487-9 (1988)
BindingDB Entry DOI: 10.7270/Q2S46QZN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50452524

(CHEMBL2372291)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C51H74N12O10S2/c1-4-73-33-19-17-32(18-20-33)26-35-44(67)59-36(25-31-13-7-5-8-14-31)46(69)62-42(30(2)3)48(71)60-37(27-40(52)64)45(68)61-38(29-74-75-51(28-41(65)57-35)21-9-6-10-22-51)49(72)63-24-12-16-39(63)47(70)58-34(43(53)66)15-11-23-56-50(54)55/h5,7-8,13-14,17-20,30,34-39,42H,4,6,9-12,15-16,21-29H2,1-3H3,(H2,52,64)(H2,53,66)(H,57,65)(H,58,70)(H,59,67)(H,60,71)(H,61,68)(H,62,69)(H4,54,55,56)/t34-,35+,36-,37-,38?,39+,42-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... |
J Med Chem 31: 1487-9 (1988)
BindingDB Entry DOI: 10.7270/Q2S46QZN |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50023754

(13-Benzyl-19-carbamoylmethyl-10-(4-ethoxy-benzyl)-...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#6]-[#6]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C54H83N15O10/c1-4-79-35-21-19-34(20-22-35)29-39-48(75)67-40(28-33-14-7-5-8-15-33)50(77)69-44(32(2)3)51(78)68-41(30-42(55)70)49(76)66-37(16-11-25-54(31-43(71)63-39)23-9-6-10-24-54)47(74)65-38(18-13-27-62-53(59)60)46(73)64-36(45(56)72)17-12-26-61-52(57)58/h5,7-8,14-15,19-22,32,36-41,44H,4,6,9-13,16-18,23-31H2,1-3H3,(H2,55,70)(H2,56,72)(H,63,71)(H,64,73)(H,65,74)(H,66,76)(H,67,75)(H,68,78)(H,69,77)(H4,57,58,61)(H4,59,60,62)/t36-,37?,38+,39-,40+,41+,44+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonist activity was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in human |
J Med Chem 31: 1487-9 (1988)
BindingDB Entry DOI: 10.7270/Q2S46QZN |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 1/2/3/4/5/6/7/8/9
(Homo sapiens (Human)) | BDBM50227472

(CHEMBL2372272)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6@H]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#6]-[#6]-[#6]-[#6](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C48H71N11O9/c1-4-68-32-19-17-31(18-20-32)26-35-43(64)57-36(25-30-13-7-5-8-14-30)45(66)59-40(29(2)3)46(67)58-37(27-38(49)60)44(65)56-34(42(63)55-33(41(50)62)16-12-24-53-47(51)52)15-11-23-48(28-39(61)54-35)21-9-6-10-22-48/h5,7-8,13-14,17-20,29,33-37,40H,4,6,9-12,15-16,21-28H2,1-3H3,(H2,49,60)(H2,50,62)(H,54,61)(H,55,63)(H,56,65)(H,57,64)(H,58,67)(H,59,66)(H4,51,52,53)/t33-,34?,35+,36-,37-,40-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... |
J Med Chem 31: 1487-9 (1988)
BindingDB Entry DOI: 10.7270/Q2S46QZN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data













