 Found 17 hits of Enzyme Inhibition Constant Data
Found 17 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM50024156

(2-(1-{[1-(2-Hydroxy-1-isobutyl-5-methyl-4-{2-methy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C50H75N9O8/c1-11-33(6)43(47(64)53-29-35-20-15-16-22-52-35)57-44(61)37(32(4)5)27-42(60)38(24-31(2)3)55-46(63)41(26-36-28-51-30-54-36)58(10)48(65)39(25-34-18-13-12-14-19-34)56-45(62)40-21-17-23-59(40)49(66)67-50(7,8)9/h12-16,18-20,22,28,30-33,37-43,60H,11,17,21,23-27,29H2,1-10H3,(H,51,54)(H,53,64)(H,55,63)(H,56,62)(H,57,61) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024151

((1-{[1-(2-Hydroxy-1-isobutyl-5-methyl-4-{2-methyl-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 |r| Show InChI InChI=1S/C45H68N8O7/c1-11-30(6)39(42(57)48-26-32-19-15-16-20-47-32)52-40(55)34(29(4)5)24-38(54)35(21-28(2)3)50-41(56)37(23-33-25-46-27-49-33)53(10)43(58)36(22-31-17-13-12-14-18-31)51-44(59)60-45(7,8)9/h12-20,25,27-30,34-39,54H,11,21-24,26H2,1-10H3,(H,46,49)(H,48,57)(H,50,56)(H,51,59)(H,52,55) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Pepsin A
(Porcine) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of porcine pepsin |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024161

((1-{1-[1-(1-Hydroxy-2-{2-methyl-1-[(pyridin-2-ylme...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccn1 |r| Show InChI InChI=1S/C43H60N6O7/c1-8-29(4)38(41(54)45-27-32-21-15-16-22-44-32)49-37(51)26-36(50)33(23-28(2)3)46-39(52)34(24-30-17-11-9-12-18-30)47-40(53)35(25-31-19-13-10-14-20-31)48-42(55)56-43(5,6)7/h9-22,28-29,33-36,38,50H,8,23-27H2,1-7H3,(H,45,54)(H,46,52)(H,47,53)(H,48,55)(H,49,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Bos taurus) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine cathepsin D |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024155

(2-{1-[(2-(3H-Imidazol-4-yl)-1-{3-methyl-1-[(2-meth...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 |r| Show InChI InChI=1S/C49H74N10O7/c1-11-33(6)42(45(62)53-28-35-20-15-16-22-51-35)57-46(63)41(32(4)5)52-29-37(24-31(2)3)55-44(61)40(26-36-27-50-30-54-36)58(10)47(64)38(25-34-18-13-12-14-19-34)56-43(60)39-21-17-23-59(39)48(65)66-49(7,8)9/h12-16,18-20,22,27,30-33,37-42,52H,11,17,21,23-26,28-29H2,1-10H3,(H,50,54)(H,53,62)(H,55,61)(H,56,60)(H,57,63) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024154

(CHEMBL3142188 | [1-({1-[1-(1-Hydroxy-2-{2-methyl-1...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccn1 |r| Show InChI InChI=1S/C44H62N6O7/c1-9-30(4)39(41(54)46-28-33-22-16-17-23-45-33)49-38(52)27-37(51)34(24-29(2)3)47-40(53)36(26-32-20-14-11-15-21-32)50(8)42(55)35(25-31-18-12-10-13-19-31)48-43(56)57-44(5,6)7/h10-23,29-30,34-37,39,51H,9,24-28H2,1-8H3,(H,46,54)(H,47,53)(H,48,56)(H,49,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024159

((1-{[1-[1-(1-Hydroxy-2-{2-methyl-1-[(pyridin-2-ylm...)Show SMILES CCC(C)C(NC(=O)CC(O)C(CC(C)C)NC(=O)C(Cc1cnc[nH]1)N(C)C(=O)C(Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C41H60N8O7/c1-9-27(4)36(38(53)44-24-29-17-13-14-18-43-29)48-35(51)22-34(50)31(19-26(2)3)46-37(52)33(21-30-23-42-25-45-30)49(8)39(54)32(20-28-15-11-10-12-16-28)47-40(55)56-41(5,6)7/h10-18,23,25-27,31-34,36,50H,9,19-22,24H2,1-8H3,(H,42,45)(H,44,53)(H,46,52)(H,47,55)(H,48,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024153

(2-(1-{[1-[1-(1-Hydroxy-2-{2-methyl-1-[(pyridin-2-y...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)OC(C)(C)C)C(=O)NCc1ccccn1 |r| Show InChI InChI=1S/C46H67N9O8/c1-9-30(4)40(43(60)49-27-32-18-13-14-20-48-32)53-39(57)25-38(56)34(22-29(2)3)51-42(59)37(24-33-26-47-28-50-33)54(8)44(61)35(23-31-16-11-10-12-17-31)52-41(58)36-19-15-21-55(36)45(62)63-46(5,6)7/h10-14,16-18,20,26,28-30,34-38,40,56H,9,15,19,21-25,27H2,1-8H3,(H,47,50)(H,49,60)(H,51,59)(H,52,58)(H,53,57) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024160

((1-{1-[1-(1-Hydroxy-2-{2-methyl-1-[(pyridin-2-ylme...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)N(C)C(=O)OC(C)(C)C)C(=O)NCc1ccccn1 |r| Show InChI InChI=1S/C44H62N6O7/c1-9-30(4)39(42(55)46-28-33-22-16-17-23-45-33)49-38(52)27-37(51)34(24-29(2)3)47-40(53)35(25-31-18-12-10-13-19-31)48-41(54)36(26-32-20-14-11-15-21-32)50(8)43(56)57-44(5,6)7/h10-23,29-30,34-37,39,51H,9,24-28H2,1-8H3,(H,46,55)(H,47,53)(H,48,54)(H,49,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024162
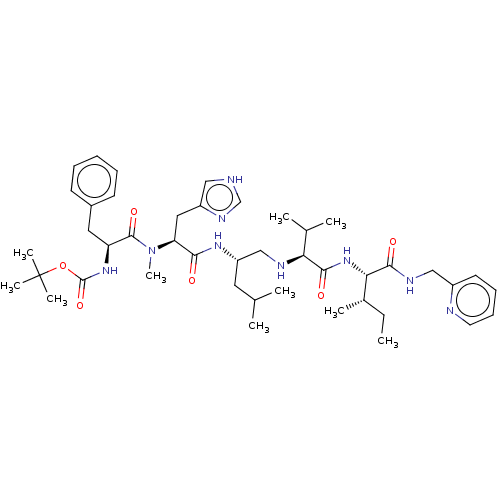
(CHEMBL3142185 | {1-[(2-(3H-Imidazol-4-yl)-1-{3-met...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 |r| Show InChI InChI=1S/C44H67N9O6/c1-11-30(6)38(40(55)48-25-32-19-15-16-20-46-32)52-41(56)37(29(4)5)47-26-34(21-28(2)3)50-39(54)36(23-33-24-45-27-49-33)53(10)42(57)35(22-31-17-13-12-14-18-31)51-43(58)59-44(7,8)9/h12-20,24,27-30,34-38,47H,11,21-23,25-26H2,1-10H3,(H,45,49)(H,48,55)(H,50,54)(H,51,58)(H,52,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024152
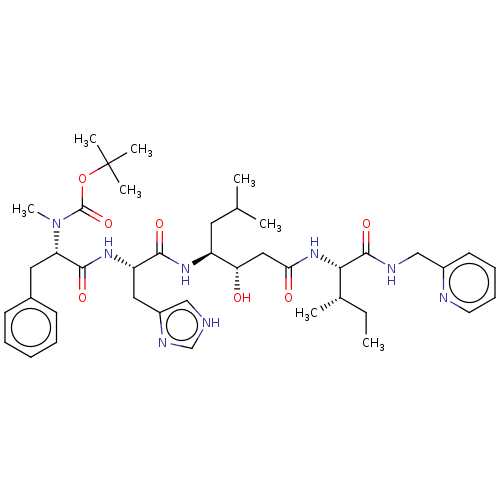
(CHEMBL3142182 | {1-[1-[1-(1-Hydroxy-2-{2-methyl-1-...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)N(C)C(=O)OC(C)(C)C)C(=O)NCc1ccccn1 |r| Show InChI InChI=1S/C41H60N8O7/c1-9-27(4)36(39(54)44-24-29-17-13-14-18-43-29)48-35(51)22-34(50)31(19-26(2)3)46-37(52)32(21-30-23-42-25-45-30)47-38(53)33(20-28-15-11-10-12-16-28)49(8)40(55)56-41(5,6)7/h10-18,23,25-27,31-34,36,50H,9,19-22,24H2,1-8H3,(H,42,45)(H,44,54)(H,46,52)(H,47,53)(H,48,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Bos taurus) | BDBM50024156

(2-(1-{[1-(2-Hydroxy-1-isobutyl-5-methyl-4-{2-methy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C50H75N9O8/c1-11-33(6)43(47(64)53-29-35-20-15-16-22-52-35)57-44(61)37(32(4)5)27-42(60)38(24-31(2)3)55-46(63)41(26-36-28-51-30-54-36)58(10)48(65)39(25-34-18-13-12-14-19-34)56-45(62)40-21-17-23-59(40)49(66)67-50(7,8)9/h12-16,18-20,22,28,30-33,37-43,60H,11,17,21,23-27,29H2,1-10H3,(H,51,54)(H,53,64)(H,55,63)(H,56,62)(H,57,61) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine cathepsin D |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Pepsin A
(Porcine) | BDBM50024156

(2-(1-{[1-(2-Hydroxy-1-isobutyl-5-methyl-4-{2-methy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C50H75N9O8/c1-11-33(6)43(47(64)53-29-35-20-15-16-22-52-35)57-44(61)37(32(4)5)27-42(60)38(24-31(2)3)55-46(63)41(26-36-28-51-30-54-36)58(10)48(65)39(25-34-18-13-12-14-19-34)56-45(62)40-21-17-23-59(40)49(66)67-50(7,8)9/h12-16,18-20,22,28,30-33,37-43,60H,11,17,21,23-27,29H2,1-10H3,(H,51,54)(H,53,64)(H,55,63)(H,56,62)(H,57,61) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of porcine pepsin |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024158

((1-{1-[1-(1-Hydroxy-2-{2-methyl-1-[(pyridin-2-ylme...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)C(C)(Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccn1 |r| Show InChI InChI=1S/C44H62N6O7/c1-9-30(4)38(40(54)46-28-33-22-16-17-23-45-33)49-37(52)26-36(51)34(24-29(2)3)47-39(53)35(25-31-18-12-10-13-19-31)48-41(55)44(8,27-32-20-14-11-15-21-32)50-42(56)57-43(5,6)7/h10-23,29-30,34-36,38,51H,9,24-28H2,1-8H3,(H,46,54)(H,47,53)(H,48,55)(H,49,52)(H,50,56) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50024157

((1-{1-[1-(1-Hydroxy-2-{2-methyl-1-[(pyridin-2-ylme...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@](C)(Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)NCc1ccccn1 |r| Show InChI InChI=1S/C44H62N6O7/c1-9-30(4)38(40(54)46-28-33-22-16-17-23-45-33)49-37(52)26-36(51)34(24-29(2)3)47-41(55)44(8,27-32-20-14-11-15-21-32)50-39(53)35(25-31-18-12-10-13-19-31)48-42(56)57-43(5,6)7/h10-23,29-30,34-36,38,51H,9,24-28H2,1-8H3,(H,46,54)(H,47,55)(H,48,56)(H,49,52)(H,50,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 29: 2088-93 (1986)
BindingDB Entry DOI: 10.7270/Q2251JRT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data