 Found 49 hits of Enzyme Inhibition Constant Data
Found 49 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024979

(3-Ethoxy-5-methyl-4,5,6,7-tetrahydro-isoxazolo[4,5...)Show InChI InChI=1S/C9H14N2O2/c1-3-12-9-7-6-11(2)5-4-8(7)13-10-9/h3-6H2,1-2H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity at pH 8.5 in rabbit lung |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024992

(3-Isopropoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]py...)Show InChI InChI=1S/C9H14N2O2/c1-6(2)12-9-7-5-10-4-3-8(7)13-11-9/h6,10H,3-5H2,1-2H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity at pH 8.5 in rabbit lung |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024980
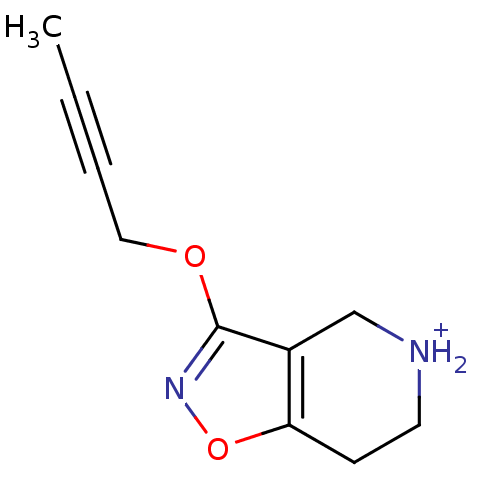
(3-But-2-ynyloxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c...)Show InChI InChI=1S/C10H12N2O2/c1-2-3-6-13-10-8-7-11-5-4-9(8)14-12-10/h11H,4-7H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of radioactive N-propylbenzilycholine mustard ([3H]-PrBCM) to rat brain membranes |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM46858
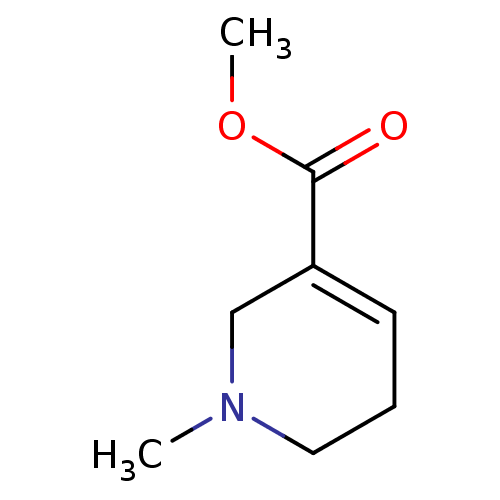
(1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...)Show InChI InChI=1S/C8H13NO2/c1-9-5-3-4-7(6-9)8(10)11-2/h4H,3,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of solubilized, purified rat liver HMG-CoA reductase. |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024995

(3-Prop-2-ynyloxy-4,5,6,7-tetrahydro-isoxazolo[4,5-...)Show InChI InChI=1S/C9H10N2O2/c1-2-5-12-9-7-6-10-4-3-8(7)13-11-9/h1,10H,3-6H2/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity in rabbit lung |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024993

(3-Ethoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyridi...)Show InChI InChI=1S/C8H12N2O2/c1-2-11-8-6-5-9-4-3-7(6)12-10-8/h9H,2-5H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of solubilized, purified rat liver HMG-CoA reductase. |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50004656

((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...)Show InChI InChI=1S/C6H14N2O2/c1-8(2,3)4-5-10-6(7)9/h4-5H2,1-3H3,(H-,7,9)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of solubilized, purified rat liver HMG-CoA reductase. |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024985

(3-Allyloxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyri...)Show InChI InChI=1S/C9H12N2O2/c1-2-5-12-9-7-6-10-4-3-8(7)13-11-9/h2,10H,1,3-6H2/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity in rabbit lung |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024990

(3-Propoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyrid...)Show InChI InChI=1S/C9H14N2O2/c1-2-5-12-9-7-6-10-4-3-8(7)13-11-9/h10H,2-6H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity in rabbit lung |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50024992

(3-Isopropoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]py...)Show InChI InChI=1S/C9H14N2O2/c1-6(2)12-9-7-5-10-4-3-8(7)13-11-9/h6,10H,3-5H2,1-2H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro antagonistic activity against peripheral Muscarinic acetylcholine receptor in guinea pig ileum as inhibition of acetylcholine induced muscle... |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024989

(3-(2-Bromo-allyloxy)-4,5,6,7-tetrahydro-isoxazolo[...)Show InChI InChI=1S/C9H11BrN2O2/c1-6(10)5-13-9-7-4-11-3-2-8(7)14-12-9/h11H,1-5H2/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity in rabbit lung |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024981

(3-But-2-enyloxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c...)Show InChI InChI=1S/C10H14N2O2/c1-2-3-6-13-10-8-7-11-5-4-9(8)14-12-10/h2-3,11H,4-7H2,1H3/p+1/b3-2+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity in rabbit lung |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024987
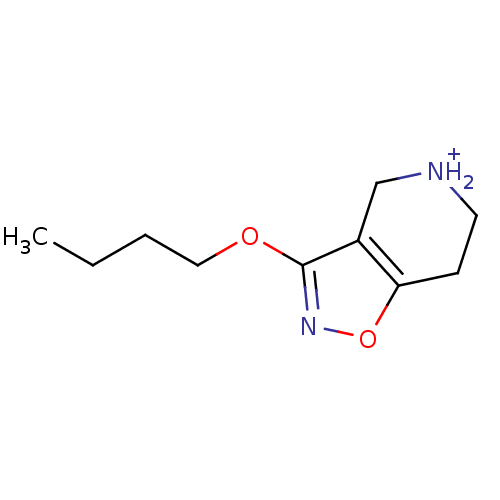
(3-Butoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyridi...)Show InChI InChI=1S/C10H16N2O2/c1-2-3-6-13-10-8-7-11-5-4-9(8)14-12-10/h11H,2-7H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of radioactive N-propylbenzilycholine mustard ([3H]-PrBCM) to rat brain membranes |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024991

(5-Ethyl-3-methoxy-4,5,6,7-tetrahydro-isoxazolo[4,5...)Show InChI InChI=1S/C9H14N2O2/c1-3-11-5-4-8-7(6-11)9(12-2)10-13-8/h3-6H2,1-2H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity at pH 8.5 in rabbit lung |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50024989

(3-(2-Bromo-allyloxy)-4,5,6,7-tetrahydro-isoxazolo[...)Show InChI InChI=1S/C9H11BrN2O2/c1-6(10)5-13-9-7-4-11-3-2-8(7)14-12-9/h11H,1-5H2/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro antagonistic activity against peripheral Muscarinic acetylcholine receptor in guinea pig ileum as inhibition of acetylcholine induced muscle... |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024996
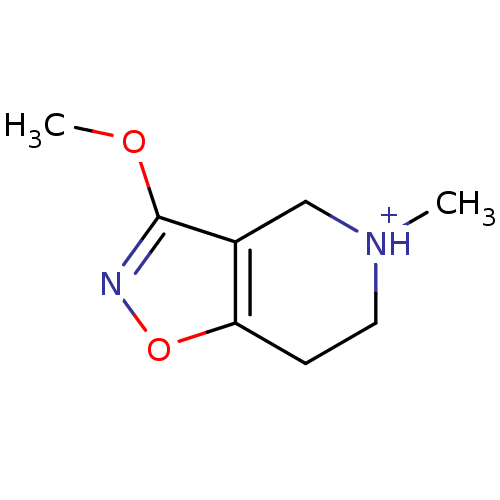
(3-Methoxy-5-methyl-4,5,6,7-tetrahydro-isoxazolo[4,...)Show InChI InChI=1S/C8H12N2O2/c1-10-4-3-7-6(5-10)8(11-2)9-12-7/h3-5H2,1-2H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of radioactive N-propylbenzilycholine mustard ([3H]-PrBCM) to rat brain membranes |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024984

(1,2,5,6-Tetrahydro-pyridine-3-carboxylic acid meth...)Show InChI InChI=1S/C7H11NO2/c1-10-7(9)6-3-2-4-8-5-6/h3,8H,2,4-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity in rabbit lung |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024983

(3-Benzyloxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyr...)Show InChI InChI=1S/C13H14N2O2/c1-2-4-10(5-3-1)9-16-13-11-8-14-7-6-12(11)17-15-13/h1-5,14H,6-9H2/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity at pH 8.5 in rabbit lung |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50024990

(3-Propoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyrid...)Show InChI InChI=1S/C9H14N2O2/c1-2-5-12-9-7-6-10-4-3-8(7)13-11-9/h10H,2-6H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro antagonistic activity against peripheral Muscarinic acetylcholine receptor in guinea pig ileum as inhibition of acetylcholine induced muscle... |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024988
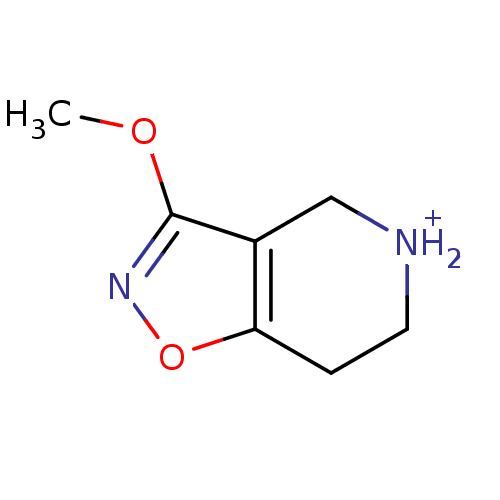
(3-Methoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyrid...)Show InChI InChI=1S/C7H10N2O2/c1-10-7-5-4-8-3-2-6(5)11-9-7/h8H,2-4H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of radioactive N-propylbenzilycholine mustard ([3H]-PrBCM) to rat brain membranes |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50024983

(3-Benzyloxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyr...)Show InChI InChI=1S/C13H14N2O2/c1-2-4-10(5-3-1)9-16-13-11-8-14-7-6-12(11)17-15-13/h1-5,14H,6-9H2/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro antagonistic activity against peripheral Muscarinic acetylcholine receptor in guinea pig ileum as inhibition of acetylcholine induced muscle... |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50024994
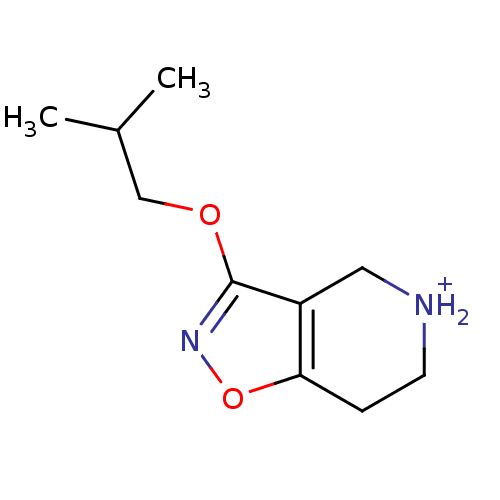
(3-Isobutoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyr...)Show InChI InChI=1S/C10H16N2O2/c1-7(2)6-13-10-8-5-11-4-3-9(8)14-12-10/h7,11H,3-6H2,1-2H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity in rabbit lung |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024986

(5-Benzyl-3-methoxy-4,5,6,7-tetrahydro-isoxazolo[4,...)Show InChI InChI=1S/C14H16N2O2/c1-17-14-12-10-16(8-7-13(12)18-15-14)9-11-5-3-2-4-6-11/h2-6H,7-10H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of solubilized, purified rat liver HMG-CoA reductase. |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024982

(3-Hydroxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyrid...)Show InChI InChI=1S/C6H8N2O2/c9-6-4-3-7-2-1-5(4)10-8-6/h7H,1-3H2,(H,8,9)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity in rabbit lung |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50024994

(3-Isobutoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyr...)Show InChI InChI=1S/C10H16N2O2/c1-7(2)6-13-10-8-5-11-4-3-9(8)14-12-10/h7,11H,3-6H2,1-2H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of binding of radioactive N-propylbenzilycholine mustard ([3H]-PrBCM) to rat brain membranes |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50024986

(5-Benzyl-3-methoxy-4,5,6,7-tetrahydro-isoxazolo[4,...)Show InChI InChI=1S/C14H16N2O2/c1-17-14-12-10-16(8-7-13(12)18-15-14)9-11-5-3-2-4-6-11/h2-6H,7-10H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro antagonistic activity against peripheral Muscarinic acetylcholine receptor in guinea pig ileum as inhibition of acetylcholine induced muscle... |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50024987

(3-Butoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyridi...)Show InChI InChI=1S/C10H16N2O2/c1-2-3-6-13-10-8-7-11-5-4-9(8)14-12-10/h11H,2-7H2,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity in rabbit lung |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50024981

(3-But-2-enyloxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c...)Show InChI InChI=1S/C10H14N2O2/c1-2-3-6-13-10-8-7-11-5-4-9(8)14-12-10/h2-3,11H,4-7H2,1H3/p+1/b3-2+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Angiotensin I converting enzyme activity in rabbit lung |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50024982

(3-Hydroxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyrid...)Show InChI InChI=1S/C6H8N2O2/c9-6-4-3-7-2-1-5(4)10-8-6/h7H,1-3H2,(H,8,9)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro antagonistic activity against peripheral Muscarinic acetylcholine receptor in guinea pig ileum as inhibition of acetylcholine induced muscle... |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024983

(3-Benzyloxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyr...)Show InChI InChI=1S/C13H14N2O2/c1-2-4-10(5-3-1)9-16-13-11-8-14-7-6-12(11)17-15-13/h1-5,14H,6-9H2/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024991

(5-Ethyl-3-methoxy-4,5,6,7-tetrahydro-isoxazolo[4,5...)Show InChI InChI=1S/C9H14N2O2/c1-3-11-5-4-8-7(6-11)9(12-2)10-13-8/h3-6H2,1-2H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024986

(5-Benzyl-3-methoxy-4,5,6,7-tetrahydro-isoxazolo[4,...)Show InChI InChI=1S/C14H16N2O2/c1-17-14-12-10-16(8-7-13(12)18-15-14)9-11-5-3-2-4-6-11/h2-6H,7-10H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024996

(3-Methoxy-5-methyl-4,5,6,7-tetrahydro-isoxazolo[4,...)Show InChI InChI=1S/C8H12N2O2/c1-10-4-3-7-6(5-10)8(11-2)9-12-7/h3-5H2,1-2H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024990

(3-Propoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyrid...)Show InChI InChI=1S/C9H14N2O2/c1-2-5-12-9-7-6-10-4-3-8(7)13-11-9/h10H,2-6H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024979

(3-Ethoxy-5-methyl-4,5,6,7-tetrahydro-isoxazolo[4,5...)Show InChI InChI=1S/C9H14N2O2/c1-3-12-9-7-6-11(2)5-4-8(7)13-10-9/h3-6H2,1-2H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024989

(3-(2-Bromo-allyloxy)-4,5,6,7-tetrahydro-isoxazolo[...)Show InChI InChI=1S/C9H11BrN2O2/c1-6(10)5-13-9-7-4-11-3-2-8(7)14-12-9/h11H,1-5H2/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50004656

((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...)Show InChI InChI=1S/C6H14N2O2/c1-8(2,3)4-5-10-6(7)9/h4-5H2,1-3H3,(H-,7,9)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM46858

(1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...)Show InChI InChI=1S/C8H13NO2/c1-9-5-3-4-7(6-9)8(10)11-2/h4H,3,5-6H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024981

(3-But-2-enyloxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c...)Show InChI InChI=1S/C10H14N2O2/c1-2-3-6-13-10-8-7-11-5-4-9(8)14-12-10/h2-3,11H,4-7H2,1H3/p+1/b3-2+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024985

(3-Allyloxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyri...)Show InChI InChI=1S/C9H12N2O2/c1-2-5-12-9-7-6-10-4-3-8(7)13-11-9/h2,10H,1,3-6H2/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024988

(3-Methoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyrid...)Show InChI InChI=1S/C7H10N2O2/c1-10-7-5-4-8-3-2-6(5)11-9-7/h8H,2-4H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024982

(3-Hydroxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyrid...)Show InChI InChI=1S/C6H8N2O2/c9-6-4-3-7-2-1-5(4)10-8-6/h7H,1-3H2,(H,8,9)/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024992

(3-Isopropoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]py...)Show InChI InChI=1S/C9H14N2O2/c1-6(2)12-9-7-5-10-4-3-8(7)13-11-9/h6,10H,3-5H2,1-2H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024995

(3-Prop-2-ynyloxy-4,5,6,7-tetrahydro-isoxazolo[4,5-...)Show InChI InChI=1S/C9H10N2O2/c1-2-5-12-9-7-6-10-4-3-8(7)13-11-9/h1,10H,3-6H2/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024987

(3-Butoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyridi...)Show InChI InChI=1S/C10H16N2O2/c1-2-3-6-13-10-8-7-11-5-4-9(8)14-12-10/h11H,2-7H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024993

(3-Ethoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyridi...)Show InChI InChI=1S/C8H12N2O2/c1-2-11-8-6-5-9-4-3-7(6)12-10-8/h9H,2-5H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024980

(3-But-2-ynyloxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c...)Show InChI InChI=1S/C10H12N2O2/c1-2-3-6-13-10-8-7-11-5-4-9(8)14-12-10/h11H,4-7H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024994

(3-Isobutoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]pyr...)Show InChI InChI=1S/C10H16N2O2/c1-7(2)6-13-10-8-5-11-4-3-9(8)14-12-10/h7,11H,3-6H2,1-2H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50024984

(1,2,5,6-Tetrahydro-pyridine-3-carboxylic acid meth...)Show InChI InChI=1S/C7H11NO2/c1-10-7(9)6-3-2-4-8-5-6/h3,8H,2,4-5H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data