

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50025927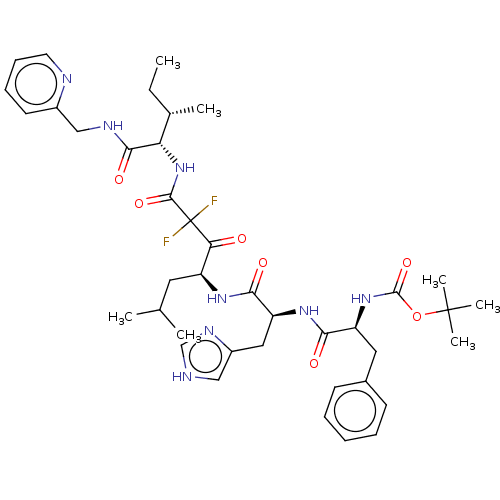 (CHEMBL3143966 | {1-[1-[1-(2,2-Difluoro-2-{2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Plasma renin inhibitory activity was evaluated in lyophilized human plasma with 0.1%EDTA | J Med Chem 28: 1553-5 (1985) BindingDB Entry DOI: 10.7270/Q23B60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50025928 (CHEMBL57121 | {1-[1-[1-(1-Hydroxy-2-{2-methyl-1-[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Plasma renin inhibitory activity was evaluated in lyophilized human plasma with 0.1%EDTA | J Med Chem 28: 1553-5 (1985) BindingDB Entry DOI: 10.7270/Q23B60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 2.0 | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against pepsin using porcine pepsin (sigma), porcine hemoglobin (sigma) and 0.02 M KCl-HCl buffer (pH 2) | J Med Chem 28: 1553-5 (1985) BindingDB Entry DOI: 10.7270/Q23B60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50025930 (CHEMBL427581 | {1-[1-[1-(2,2-Difluoro-1-hydroxy-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Plasma renin inhibitory activity was evaluated in lyophilized human plasma with 0.1%EDTA | J Med Chem 28: 1553-5 (1985) BindingDB Entry DOI: 10.7270/Q23B60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Bos taurus) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against bovine cathepsin D (sigma) | J Med Chem 28: 1553-5 (1985) BindingDB Entry DOI: 10.7270/Q23B60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50025926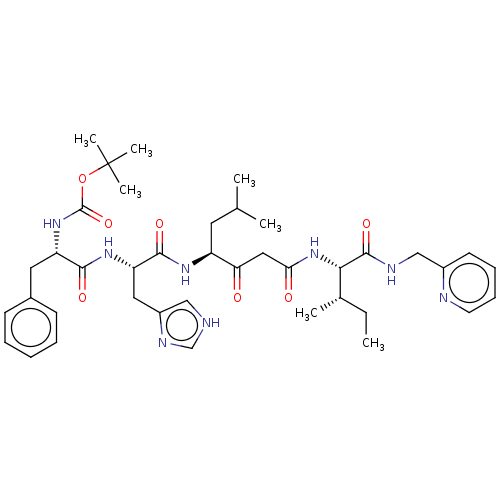 ((1-{2-(1H-Imidazol-4-yl)-1-[3-methyl-1-(2-{2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Plasma renin inhibitory activity was evaluated in lyophilized human plasma with 0.1%EDTA | J Med Chem 28: 1553-5 (1985) BindingDB Entry DOI: 10.7270/Q23B60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50025929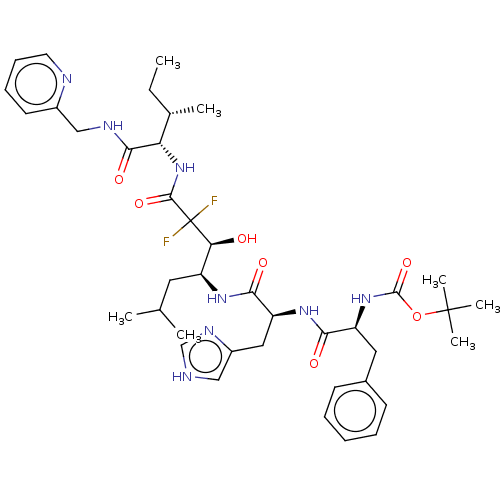 (CHEMBL57399 | {1-[1-[1-(2,2-Difluoro-1-hydroxy-2-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Plasma renin inhibitory activity was evaluated in lyophilized human plasma with 0.1%EDTA | J Med Chem 28: 1553-5 (1985) BindingDB Entry DOI: 10.7270/Q23B60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Plasma renin inhibitory activity was evaluated in lyophilized human plasma with 0.1%EDTA | J Med Chem 28: 1553-5 (1985) BindingDB Entry DOI: 10.7270/Q23B60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022851 (CHEMBL413534 | Pro-His-Pro-Phe-His-Phe-Phe-Val-Tyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Plasma renin inhibitory activity was evaluated in lyophilized human plasma with 0.1%EDTA | J Med Chem 28: 1553-5 (1985) BindingDB Entry DOI: 10.7270/Q23B60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50022851 (CHEMBL413534 | Pro-His-Pro-Phe-His-Phe-Phe-Val-Tyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against Angiotensin I converting enzyme activity in rabbit | J Med Chem 28: 1553-5 (1985) BindingDB Entry DOI: 10.7270/Q23B60QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||