

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM318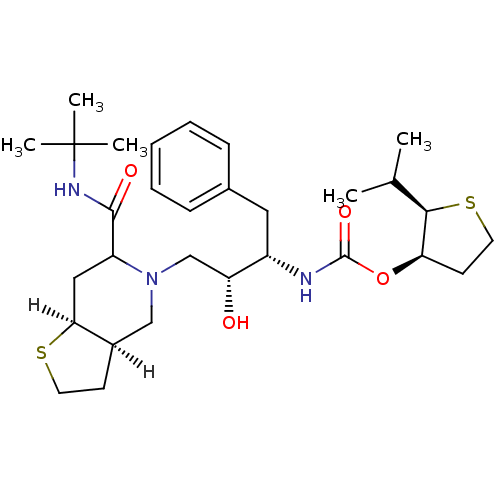 ((2R,3R)-2-(propan-2-yl)thiolan-3-yl N-[(2S,3R)-4-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285971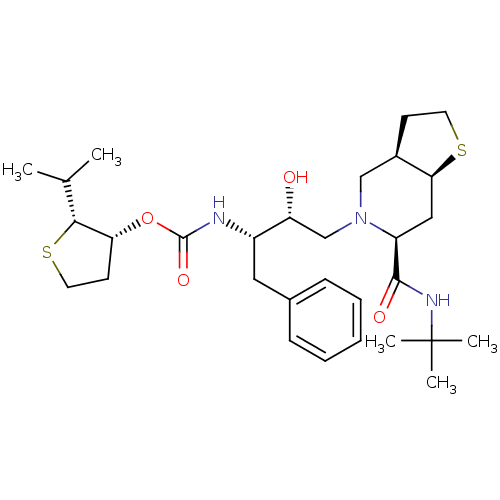 (CHEMBL95546 | [(1S,2R)-1-Benzyl-3-((3aR,6S,7aS)-6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM306 ((2S,3S)-2-methyloxolan-3-yl N-[(2R,3R)-4-[(3aR,7aS...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285969 (CHEMBL99336 | [(1R,2R)-3-((3aR,6S,7aS)-6-tert-Buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285976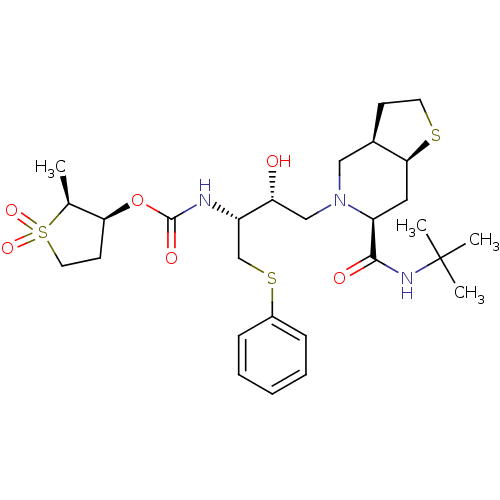 (CHEMBL321949 | [(1R,2R)-3-((3aR,6S,7aS)-6-tert-But...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM313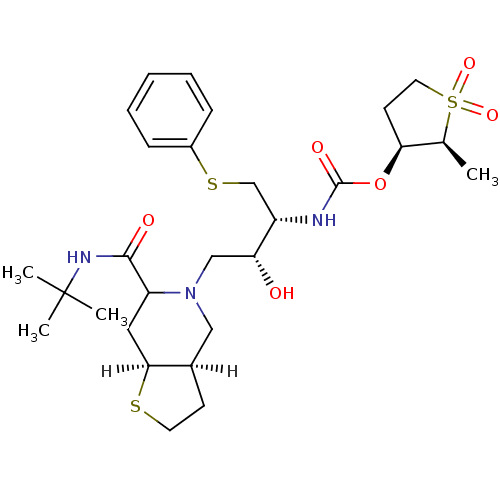 ((2S,3S)-2-methyl-1,1-dioxo--thiolan-3-yl N-[(2R,3R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285980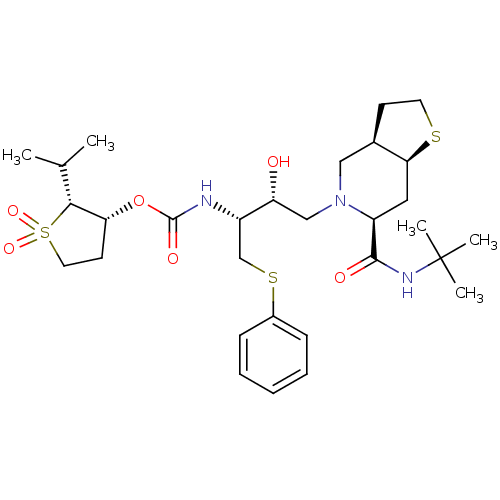 (CHEMBL97585 | LY-326188 | [(1R,2R)-3-((3aR,6S,7aS)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM322 ((2R,3R)-1,1-dioxo-2-(propan-2-yl)--thiolan-3-yl N-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM314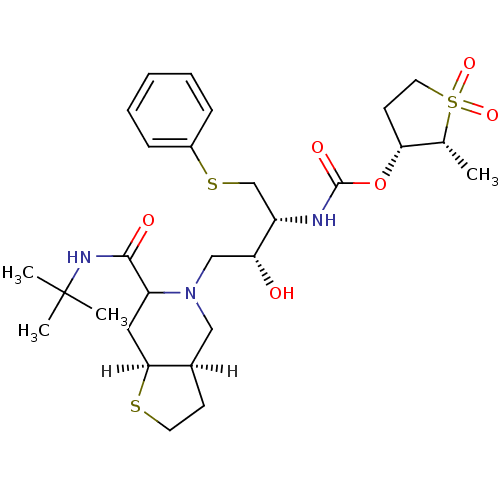 ((2R,3R)-2-methyl-1,1-dioxo--thiolan-3-yl N-[(2R,3R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285977 (CHEMBL99214 | [(1R,2R)-3-((3aR,6S,7aS)-6-tert-Buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285982 (CHEMBL316717 | [(1R,2R)-3-((3aR,6S,7aS)-6-tert-But...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM321 ((2S,3S)-1,1-dioxo-2-(propan-2-yl)--thiolan-3-yl N-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285975 (CHEMBL316718 | [(1S,2R)-1-Benzyl-3-((3aR,6S,7aS)-6...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM320 ((2R,3R)-1,1-dioxo-2-(propan-2-yl)--thiolan-3-yl N-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285970 (CHEMBL318129 | [(1R,2R)-3-((3aR,6S,7aS)-6-tert-But...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM312 ((2S,3R)-2-methyl-1,1-dioxo--thiolan-3-yl N-[(2R,3R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285974 (CHEMBL100048 | [(1R,2R)-3-((3aR,6S,7aS)-6-tert-But...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM308 ((3S)-1,1-dioxo--thiolan-3-yl N-[(2R,3R)-4-[(3aR,7a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285979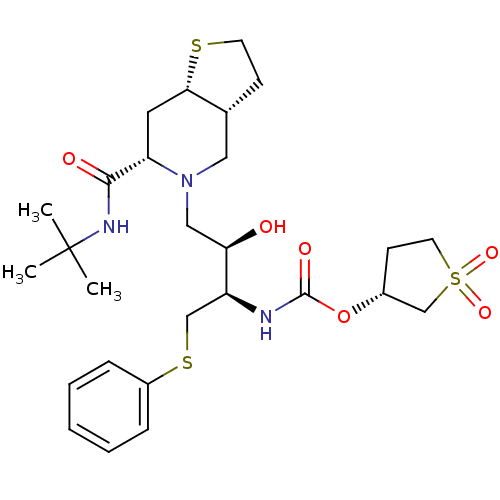 (CHEMBL316608 | [(1R,2R)-3-((3aR,6S,7aS)-6-tert-But...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM309 ((3R)-1,1-dioxo--thiolan-3-yl N-[(2R,3R)-4-[(3aR,7a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM319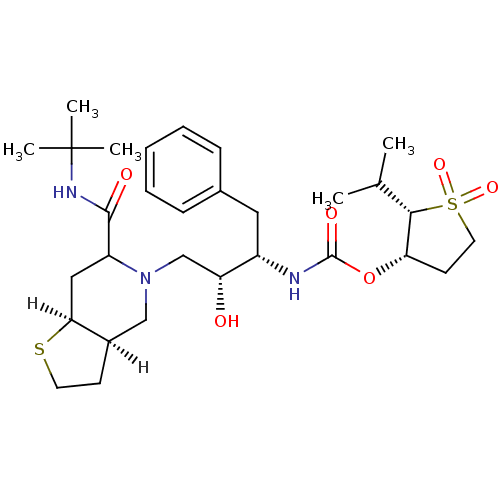 ((2S,3S)-1,1-dioxo-2-(propan-2-yl)--thiolan-3-yl N-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM317 ((2S,3S)-2-(propan-2-yl)thiolan-3-yl N-[(2S,3R)-4-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285972 (CHEMBL321778 | [(1S,2R)-1-Benzyl-3-((3aR,6S,7aS)-6...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285978 (CHEMBL100271 | [(1S,2R)-1-Benzyl-3-((3aR,6S,7aS)-6...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285981 (CHEMBL318894 | [(1R,2R)-3-((3aR,6S,7aS)-6-tert-But...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM307 ((2R,3R)-2-methyloxolan-3-yl N-[(2R,3R)-4-[(3aR,7aS...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM316 ((2R,3R)-1,1-dioxo-2-(propan-2-yl)--thiolan-3-yl N-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285968 (CHEMBL18067 | [(1S,2R)-1-Benzyl-3-((3S,4aS,8aS)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM311 ((2R,3S)-2-methyl-1,1-dioxo--thiolan-3-yl N-[(2R,3R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285967 (CHEMBL320602 | [(1R,2R)-3-((3aR,6S,7aS)-6-tert-But...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM315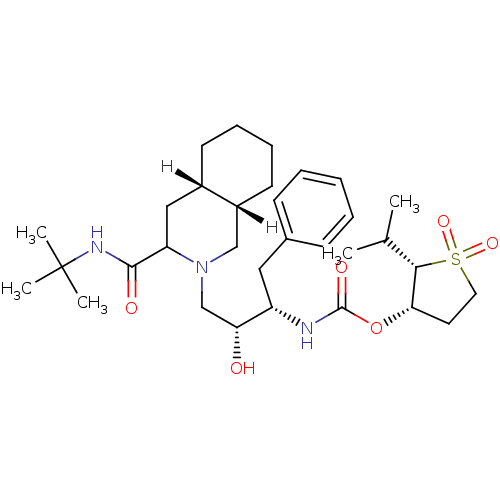 ((2S,3S)-1,1-dioxo-2-(propan-2-yl)--thiolan-3-yl N-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285973 (CHEMBL261340 | [(1S,2R)-1-Benzyl-3-((3S,4aS,8aS)-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM310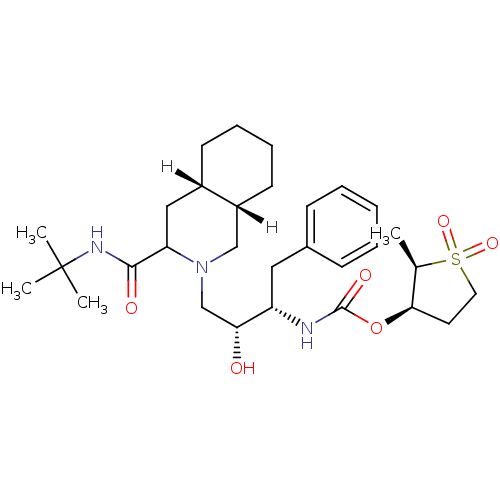 ((2R,3R)-2-methyl-1,1-dioxo--thiolan-3-yl N-[(2S,3R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 5.5 | 22 |
Lilly Research Laboratories | Assay Description The assay involves the use of a HIV-1 protease peptide substrate which has been modified to contain a biotin moiety on one side and a fluorescent rep... | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM532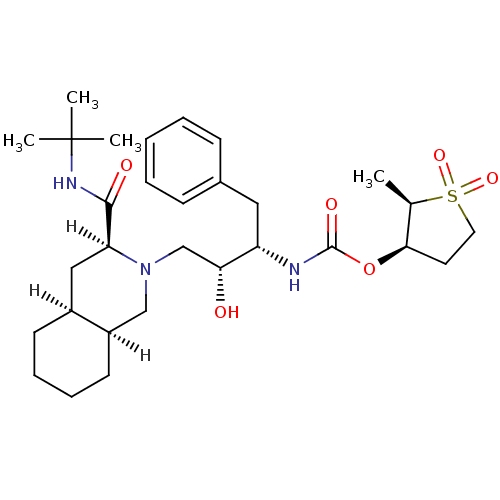 ((2R,3R)-2-methyl-1,1-dioxo--thiolan-3-yl N-[(2S,3R...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories | Assay Description In vitro inhibition of HIV-1 protease. | Bioorg Med Chem Lett 5: 2891-6 (1995) Article DOI: 10.1016/0960-894X(95)00507-P BindingDB Entry DOI: 10.7270/Q2T43R7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||