

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50280489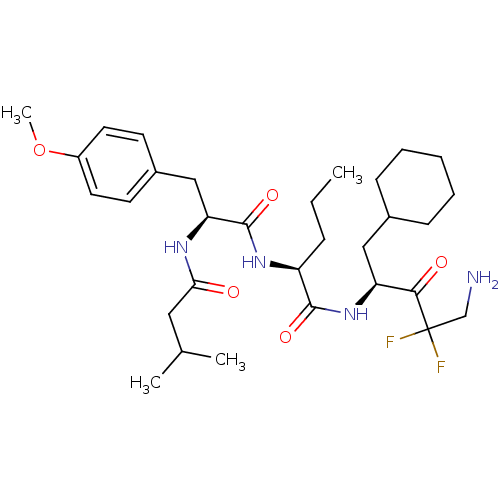 ((S)-2-[(S)-3-(4-Methoxy-phenyl)-2-(3-methyl-butyry...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit the enzyme chymotrypsin | Bioorg Med Chem Lett 2: 651-654 (1992) Article DOI: 10.1016/S0960-894X(00)80383-2 BindingDB Entry DOI: 10.7270/Q2KP822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50280489 ((S)-2-[(S)-3-(4-Methoxy-phenyl)-2-(3-methyl-butyry...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit the enzyme cathepsin | Bioorg Med Chem Lett 2: 651-654 (1992) Article DOI: 10.1016/S0960-894X(00)80383-2 BindingDB Entry DOI: 10.7270/Q2KP822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50280489 ((S)-2-[(S)-3-(4-Methoxy-phenyl)-2-(3-methyl-butyry...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit the enzyme pepsin | Bioorg Med Chem Lett 2: 651-654 (1992) Article DOI: 10.1016/S0960-894X(00)80383-2 BindingDB Entry DOI: 10.7270/Q2KP822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50280489 ((S)-2-[(S)-3-(4-Methoxy-phenyl)-2-(3-methyl-butyry...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit human plasma renin by 50% using radio-immunoassay was determined | Bioorg Med Chem Lett 2: 651-654 (1992) Article DOI: 10.1016/S0960-894X(00)80383-2 BindingDB Entry DOI: 10.7270/Q2KP822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50280489 ((S)-2-[(S)-3-(4-Methoxy-phenyl)-2-(3-methyl-butyry...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for the antagonistic activity against 5-hydroxytryptamine 3 receptor in isolated perfused rabbit heart (RH) | Bioorg Med Chem Lett 2: 651-654 (1992) Article DOI: 10.1016/S0960-894X(00)80383-2 BindingDB Entry DOI: 10.7270/Q2KP822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50280493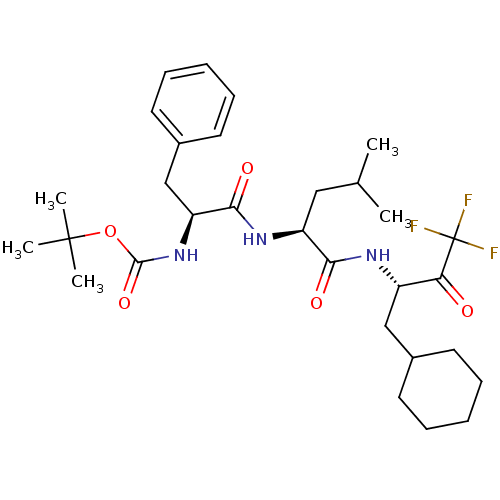 (CHEMBL430077 | {(S)-1-[(S)-1-((S)-1-Cyclohexylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit human plasma renin by 50% using radio-immunoassay was determined | Bioorg Med Chem Lett 2: 651-654 (1992) Article DOI: 10.1016/S0960-894X(00)80383-2 BindingDB Entry DOI: 10.7270/Q2KP822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50280490 ((S)-2-[(S)-3-(4-Methoxy-phenyl)-2-(3-methyl-butyry...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit human plasma renin by 50% using radio-immunoassay was determined | Bioorg Med Chem Lett 2: 651-654 (1992) Article DOI: 10.1016/S0960-894X(00)80383-2 BindingDB Entry DOI: 10.7270/Q2KP822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50280489 ((S)-2-[(S)-3-(4-Methoxy-phenyl)-2-(3-methyl-butyry...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated for the antagonistic activity against 5-hydroxytryptamine 3 receptor in isolated guinea pig ileum (GPI) | Bioorg Med Chem Lett 2: 651-654 (1992) Article DOI: 10.1016/S0960-894X(00)80383-2 BindingDB Entry DOI: 10.7270/Q2KP822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50280491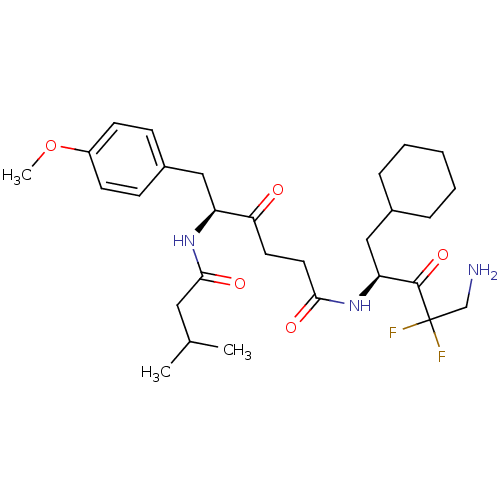 ((S)-6-(4-Methoxy-phenyl)-5-(3-methyl-butyrylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit human plasma renin by 50% using radio-immunoassay was determined | Bioorg Med Chem Lett 2: 651-654 (1992) Article DOI: 10.1016/S0960-894X(00)80383-2 BindingDB Entry DOI: 10.7270/Q2KP822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50280494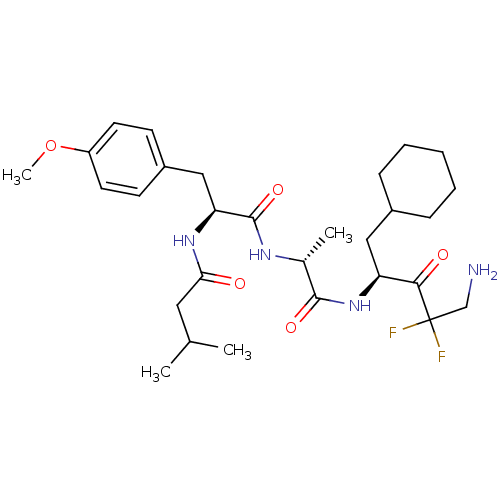 (CHEMBL545117 | N-[(S)-1-[(R)-1-((S)-4-Amino-1-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit human plasma renin by 50% using radio-immunoassay was determined | Bioorg Med Chem Lett 2: 651-654 (1992) Article DOI: 10.1016/S0960-894X(00)80383-2 BindingDB Entry DOI: 10.7270/Q2KP822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50280492 ((S)-2-{[(S)-3-(4-Methoxy-phenyl)-2-(3-methyl-butyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit human plasma renin by 50% using radio-immunoassay was determined | Bioorg Med Chem Lett 2: 651-654 (1992) Article DOI: 10.1016/S0960-894X(00)80383-2 BindingDB Entry DOI: 10.7270/Q2KP822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Rattus norvegicus) | BDBM50280489 ((S)-2-[(S)-3-(4-Methoxy-phenyl)-2-(3-methyl-butyry...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit rat plasma renin by 50% using radio-immunoassay was determined | Bioorg Med Chem Lett 2: 651-654 (1992) Article DOI: 10.1016/S0960-894X(00)80383-2 BindingDB Entry DOI: 10.7270/Q2KP822J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||