 Found 18 hits of Enzyme Inhibition Constant Data
Found 18 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50280660
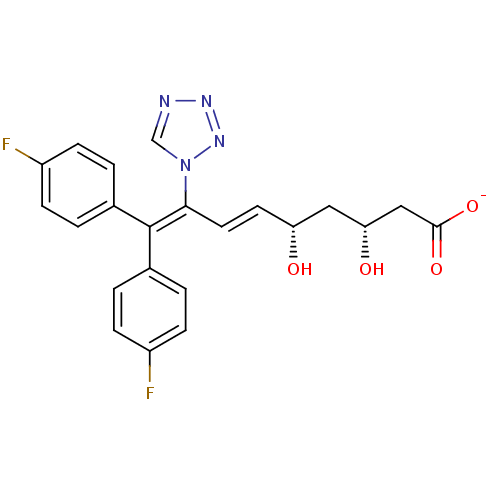
(CHEMBL11891 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluor...)Show SMILES [#8]-[#6@H](-[#6]-[#6@H](-[#8])\[#6]=[#6]\[#6](=[#6](/c1ccc(F)cc1)-c1ccc(F)cc1)\n1cnnn1)-[#6]-[#6](-[#8-])=O Show InChI InChI=1S/C22H20F2N4O4/c23-16-5-1-14(2-6-16)22(15-3-7-17(24)8-4-15)20(28-13-25-26-27-28)10-9-18(29)11-19(30)12-21(31)32/h1-10,13,18-19,29-30H,11-12H2,(H,31,32)/p-1/b10-9+/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against HMG-CoA reductase from rat hepatocyte |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50014345
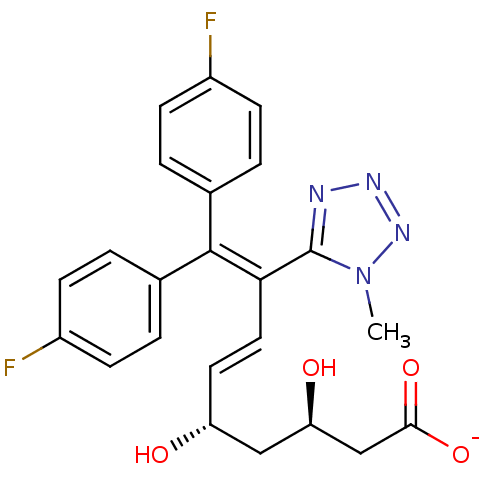
(CHEMBL11804 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluor...)Show SMILES [#6]-n1nnnc1\[#6](\[#6]=[#6]\[#6@@H](-[#8])-[#6]-[#6@@H](-[#8])-[#6]-[#6](-[#8-])=O)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N4O4/c1-29-23(26-27-28-29)20(11-10-18(30)12-19(31)13-21(32)33)22(14-2-6-16(24)7-3-14)15-4-8-17(25)9-5-15/h2-11,18-19,30-31H,12-13H2,1H3,(H,32,33)/p-1/b11-10+/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against HMG-CoA reductase in HepG2 cells |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50014345

(CHEMBL11804 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluor...)Show SMILES [#6]-n1nnnc1\[#6](\[#6]=[#6]\[#6@@H](-[#8])-[#6]-[#6@@H](-[#8])-[#6]-[#6](-[#8-])=O)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N4O4/c1-29-23(26-27-28-29)20(11-10-18(30)12-19(31)13-21(32)33)22(14-2-6-16(24)7-3-14)15-4-8-17(25)9-5-15/h2-11,18-19,30-31H,12-13H2,1H3,(H,32,33)/p-1/b11-10+/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against HMG-CoA reductase in liver cell preparation |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Mus musculus) | BDBM50004774
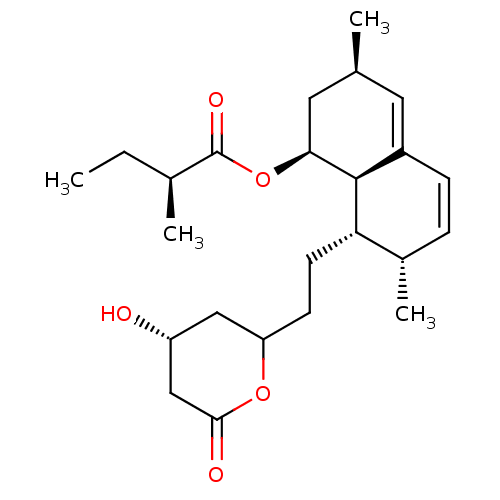
((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibitory activity against isolated enzyme HMG-CoA reductase |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against HMG-CoA reductase from rat hepatocyte |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50004774

((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against HMG-CoA reductase in HepG2 cells |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Mus musculus) | BDBM50014345

(CHEMBL11804 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluor...)Show SMILES [#6]-n1nnnc1\[#6](\[#6]=[#6]\[#6@@H](-[#8])-[#6]-[#6@@H](-[#8])-[#6]-[#6](-[#8-])=O)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N4O4/c1-29-23(26-27-28-29)20(11-10-18(30)12-19(31)13-21(32)33)22(14-2-6-16(24)7-3-14)15-4-8-17(25)9-5-15/h2-11,18-19,30-31H,12-13H2,1H3,(H,32,33)/p-1/b11-10+/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibitory activity against isolated enzyme HMG-CoA reductase |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50280661
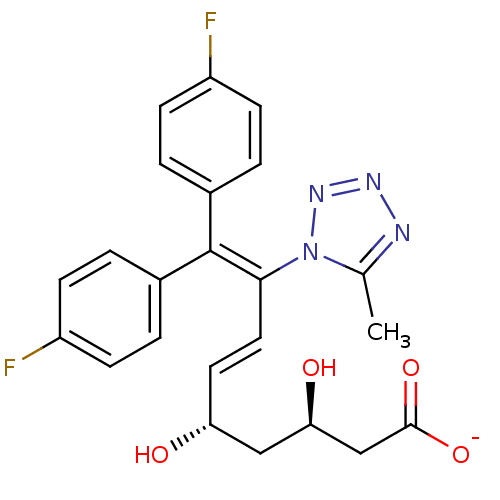
(CHEMBL276717 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluo...)Show SMILES [#6]-c1nnnn1\[#6](\[#6]=[#6]\[#6@@H](-[#8])-[#6]-[#6@@H](-[#8])-[#6]-[#6](-[#8-])=O)=[#6](/c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N4O4/c1-14-26-27-28-29(14)21(11-10-19(30)12-20(31)13-22(32)33)23(15-2-6-17(24)7-3-15)16-4-8-18(25)9-5-16/h2-11,19-20,30-31H,12-13H2,1H3,(H,32,33)/p-1/b11-10+/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against HMG-CoA reductase from rat hepatocyte |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Mus musculus) | BDBM50280660

(CHEMBL11891 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluor...)Show SMILES [#8]-[#6@H](-[#6]-[#6@H](-[#8])\[#6]=[#6]\[#6](=[#6](/c1ccc(F)cc1)-c1ccc(F)cc1)\n1cnnn1)-[#6]-[#6](-[#8-])=O Show InChI InChI=1S/C22H20F2N4O4/c23-16-5-1-14(2-6-16)22(15-3-7-17(24)8-4-15)20(28-13-25-26-27-28)10-9-18(29)11-19(30)12-21(31)32/h1-10,13,18-19,29-30H,11-12H2,(H,31,32)/p-1/b10-9+/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibitory activity against isolated enzyme HMG-CoA reductase |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Mus musculus) | BDBM50280661

(CHEMBL276717 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluo...)Show SMILES [#6]-c1nnnn1\[#6](\[#6]=[#6]\[#6@@H](-[#8])-[#6]-[#6@@H](-[#8])-[#6]-[#6](-[#8-])=O)=[#6](/c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N4O4/c1-14-26-27-28-29(14)21(11-10-19(30)12-20(31)13-22(32)33)23(15-2-6-17(24)7-3-15)16-4-8-18(25)9-5-16/h2-11,19-20,30-31H,12-13H2,1H3,(H,32,33)/p-1/b11-10+/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibitory activity against isolated enzyme HMG-CoA reductase |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50280661

(CHEMBL276717 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluo...)Show SMILES [#6]-c1nnnn1\[#6](\[#6]=[#6]\[#6@@H](-[#8])-[#6]-[#6@@H](-[#8])-[#6]-[#6](-[#8-])=O)=[#6](/c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N4O4/c1-14-26-27-28-29(14)21(11-10-19(30)12-20(31)13-22(32)33)23(15-2-6-17(24)7-3-15)16-4-8-18(25)9-5-16/h2-11,19-20,30-31H,12-13H2,1H3,(H,32,33)/p-1/b11-10+/t19-,20-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against HMG-CoA reductase in HepG2 cells |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50014345

(CHEMBL11804 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluor...)Show SMILES [#6]-n1nnnc1\[#6](\[#6]=[#6]\[#6@@H](-[#8])-[#6]-[#6@@H](-[#8])-[#6]-[#6](-[#8-])=O)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N4O4/c1-29-23(26-27-28-29)20(11-10-18(30)12-19(31)13-21(32)33)22(14-2-6-16(24)7-3-14)15-4-8-17(25)9-5-15/h2-11,18-19,30-31H,12-13H2,1H3,(H,32,33)/p-1/b11-10+/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against HMG-CoA reductase in testes cell preparation |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50014345

(CHEMBL11804 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluor...)Show SMILES [#6]-n1nnnc1\[#6](\[#6]=[#6]\[#6@@H](-[#8])-[#6]-[#6@@H](-[#8])-[#6]-[#6](-[#8-])=O)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N4O4/c1-29-23(26-27-28-29)20(11-10-18(30)12-19(31)13-21(32)33)22(14-2-6-16(24)7-3-14)15-4-8-17(25)9-5-15/h2-11,18-19,30-31H,12-13H2,1H3,(H,32,33)/p-1/b11-10+/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against HMG-CoA reductase in adrenal cell preparation |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50280660

(CHEMBL11891 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluor...)Show SMILES [#8]-[#6@H](-[#6]-[#6@H](-[#8])\[#6]=[#6]\[#6](=[#6](/c1ccc(F)cc1)-c1ccc(F)cc1)\n1cnnn1)-[#6]-[#6](-[#8-])=O Show InChI InChI=1S/C22H20F2N4O4/c23-16-5-1-14(2-6-16)22(15-3-7-17(24)8-4-15)20(28-13-25-26-27-28)10-9-18(29)11-19(30)12-21(31)32/h1-10,13,18-19,29-30H,11-12H2,(H,31,32)/p-1/b10-9+/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against HMG-CoA reductase in HepG2 cells |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50014345

(CHEMBL11804 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluor...)Show SMILES [#6]-n1nnnc1\[#6](\[#6]=[#6]\[#6@@H](-[#8])-[#6]-[#6@@H](-[#8])-[#6]-[#6](-[#8-])=O)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N4O4/c1-29-23(26-27-28-29)20(11-10-18(30)12-19(31)13-21(32)33)22(14-2-6-16(24)7-3-14)15-4-8-17(25)9-5-15/h2-11,18-19,30-31H,12-13H2,1H3,(H,32,33)/p-1/b11-10+/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against HMG-CoA reductase in bovine occular cell preparation |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50014345

(CHEMBL11804 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluor...)Show SMILES [#6]-n1nnnc1\[#6](\[#6]=[#6]\[#6@@H](-[#8])-[#6]-[#6@@H](-[#8])-[#6]-[#6](-[#8-])=O)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N4O4/c1-29-23(26-27-28-29)20(11-10-18(30)12-19(31)13-21(32)33)22(14-2-6-16(24)7-3-14)15-4-8-17(25)9-5-15/h2-11,18-19,30-31H,12-13H2,1H3,(H,32,33)/p-1/b11-10+/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against rat hepatocyte |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50014345

(CHEMBL11804 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluor...)Show SMILES [#6]-n1nnnc1\[#6](\[#6]=[#6]\[#6@@H](-[#8])-[#6]-[#6@@H](-[#8])-[#6]-[#6](-[#8-])=O)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N4O4/c1-29-23(26-27-28-29)20(11-10-18(30)12-19(31)13-21(32)33)22(14-2-6-16(24)7-3-14)15-4-8-17(25)9-5-15/h2-11,18-19,30-31H,12-13H2,1H3,(H,32,33)/p-1/b11-10+/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against HMG-CoA reductase in spleen cell preparation |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Mus musculus) | BDBM50280662
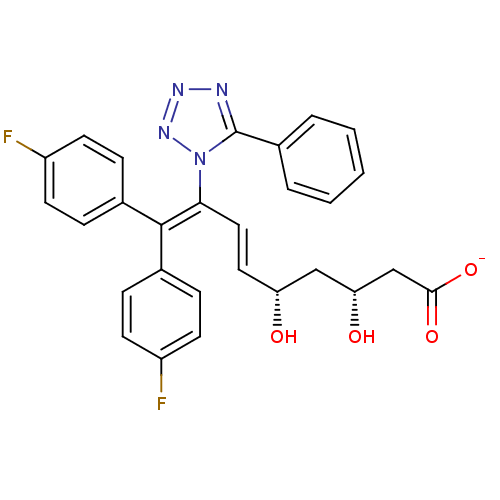
(CHEMBL268767 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluo...)Show SMILES [#8]-[#6@H](-[#6]-[#6@H](-[#8])\[#6]=[#6]\[#6](=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1)\n1nnnc1-c1ccccc1)-[#6]-[#6](-[#8-])=O Show InChI InChI=1S/C28H24F2N4O4/c29-21-10-6-18(7-11-21)27(19-8-12-22(30)13-9-19)25(15-14-23(35)16-24(36)17-26(37)38)34-28(31-32-33-34)20-4-2-1-3-5-20/h1-15,23-24,35-36H,16-17H2,(H,37,38)/p-1/b15-14+/t23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibitory activity against isolated enzyme HMG-CoA reductase |
Bioorg Med Chem Lett 2: 1085-1088 (1992)
Article DOI: 10.1016/S0960-894X(00)80623-X
BindingDB Entry DOI: 10.7270/Q2NG4QJQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data