 Found 11 hits of Enzyme Inhibition Constant Data
Found 11 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50288056
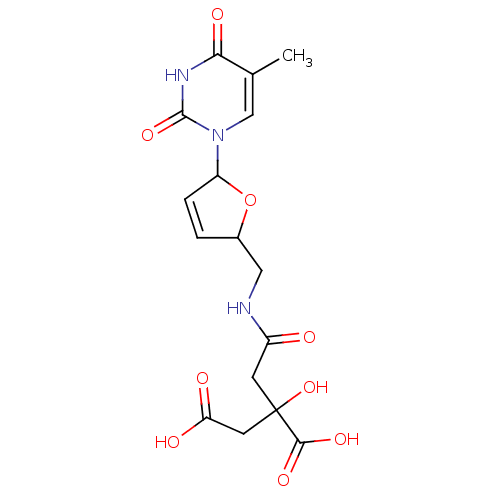
(2-Hydroxy-2-({[5-(5-methyl-2,4-dioxo-3,4-dihydro-2...)Show SMILES Cc1cn(C2OC(CNC(=O)CC(O)(CC(O)=O)C(O)=O)C=C2)c(=O)[nH]c1=O |c:21| Show InChI InChI=1S/C16H19N3O9/c1-8-7-19(15(26)18-13(8)23)11-3-2-9(28-11)6-17-10(20)4-16(27,14(24)25)5-12(21)22/h2-3,7,9,11,27H,4-6H2,1H3,(H,17,20)(H,21,22)(H,24,25)(H,18,23,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
Bioorg Med Chem Lett 6: 2405-2410 (1996)
Article DOI: 10.1016/0960-894X(96)00444-1
BindingDB Entry DOI: 10.7270/Q2Z31ZMC |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50288062
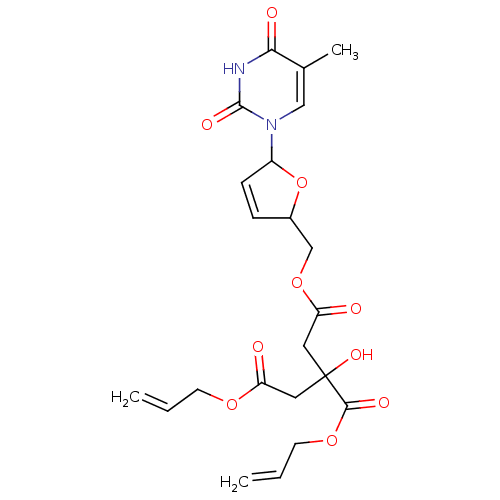
(3-Allyloxycarbonyl-3-hydroxy-pentanedioic acid all...)Show SMILES Cc1cn(C2OC(COC(=O)CC(O)(CC(=O)OCC=C)C(=O)OCC=C)C=C2)c(=O)[nH]c1=O |c:27| Show InChI InChI=1S/C22H26N2O10/c1-4-8-31-17(25)10-22(30,20(28)32-9-5-2)11-18(26)33-13-15-6-7-16(34-15)24-12-14(3)19(27)23-21(24)29/h4-7,12,15-16,30H,1-2,8-11,13H2,3H3,(H,23,27,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
Bioorg Med Chem Lett 6: 2405-2410 (1996)
Article DOI: 10.1016/0960-894X(96)00444-1
BindingDB Entry DOI: 10.7270/Q2Z31ZMC |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50288059
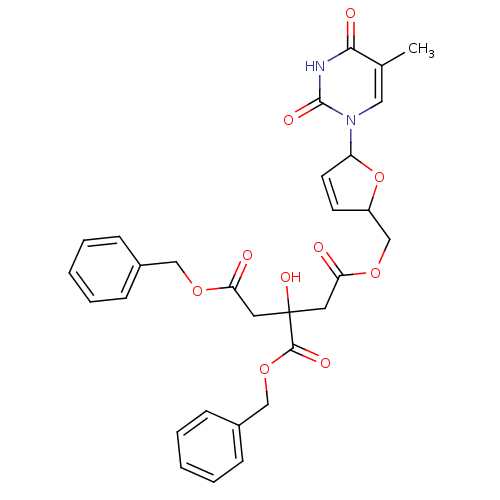
(3-Benzyloxycarbonyl-3-hydroxy-pentanedioic acid be...)Show SMILES Cc1cn(C2OC(COC(=O)CC(O)(CC(=O)OCc3ccccc3)C(=O)OCc3ccccc3)C=C2)c(=O)[nH]c1=O |c:37| Show InChI InChI=1S/C30H30N2O10/c1-20-16-32(29(37)31-27(20)35)24-13-12-23(42-24)19-40-26(34)15-30(38,28(36)41-18-22-10-6-3-7-11-22)14-25(33)39-17-21-8-4-2-5-9-21/h2-13,16,23-24,38H,14-15,17-19H2,1H3,(H,31,35,37) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
Bioorg Med Chem Lett 6: 2405-2410 (1996)
Article DOI: 10.1016/0960-894X(96)00444-1
BindingDB Entry DOI: 10.7270/Q2Z31ZMC |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50288061
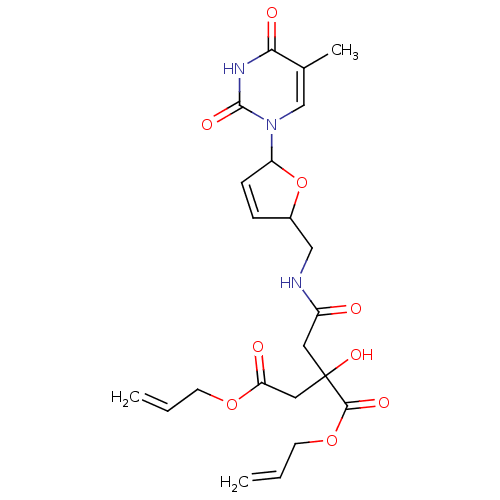
(2-Hydroxy-2-({[5-(5-methyl-2,4-dioxo-3,4-dihydro-2...)Show SMILES Cc1cn(C2OC(CNC(=O)CC(O)(CC(=O)OCC=C)C(=O)OCC=C)C=C2)c(=O)[nH]c1=O |c:27| Show InChI InChI=1S/C22H27N3O9/c1-4-8-32-18(27)11-22(31,20(29)33-9-5-2)10-16(26)23-12-15-6-7-17(34-15)25-13-14(3)19(28)24-21(25)30/h4-7,13,15,17,31H,1-2,8-12H2,3H3,(H,23,26)(H,24,28,30) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
Bioorg Med Chem Lett 6: 2405-2410 (1996)
Article DOI: 10.1016/0960-894X(96)00444-1
BindingDB Entry DOI: 10.7270/Q2Z31ZMC |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50288055
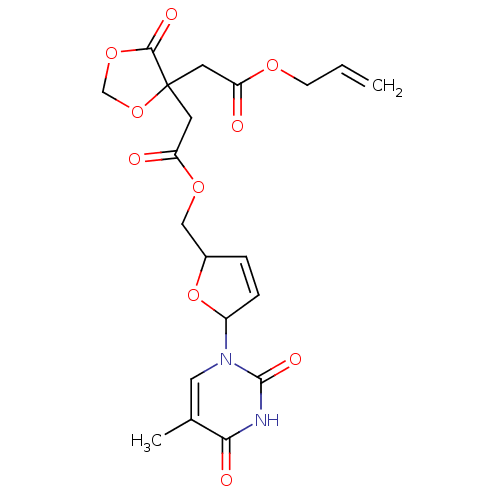
((4-Allyloxycarbonylmethyl-5-oxo-[1,3]dioxolan-4-yl...)Show SMILES Cc1cn(C2OC(COC(=O)CC3(CC(=O)OCC=C)OCOC3=O)C=C2)c(=O)[nH]c1=O |c:26| Show InChI InChI=1S/C20H22N2O10/c1-3-6-28-15(23)7-20(18(26)30-11-31-20)8-16(24)29-10-13-4-5-14(32-13)22-9-12(2)17(25)21-19(22)27/h3-5,9,13-14H,1,6-8,10-11H2,2H3,(H,21,25,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
Bioorg Med Chem Lett 6: 2405-2410 (1996)
Article DOI: 10.1016/0960-894X(96)00444-1
BindingDB Entry DOI: 10.7270/Q2Z31ZMC |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50288060
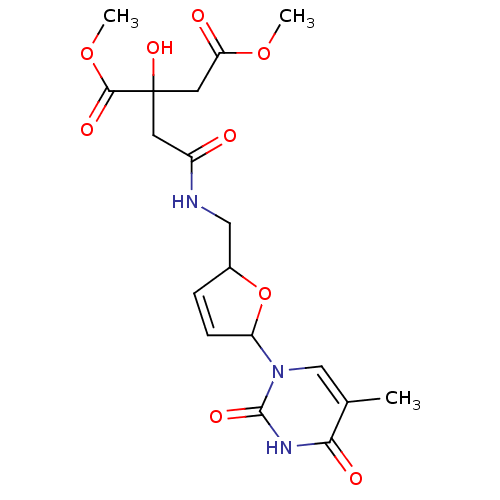
(2-Hydroxy-2-({[5-(5-methyl-2,4-dioxo-3,4-dihydro-2...)Show SMILES COC(=O)CC(O)(CC(=O)NCC1OC(C=C1)n1cc(C)c(=O)[nH]c1=O)C(=O)OC |c:15| Show InChI InChI=1S/C18H23N3O9/c1-10-9-21(17(26)20-15(10)24)13-5-4-11(30-13)8-19-12(22)6-18(27,16(25)29-3)7-14(23)28-2/h4-5,9,11,13,27H,6-8H2,1-3H3,(H,19,22)(H,20,24,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
Bioorg Med Chem Lett 6: 2405-2410 (1996)
Article DOI: 10.1016/0960-894X(96)00444-1
BindingDB Entry DOI: 10.7270/Q2Z31ZMC |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50214280
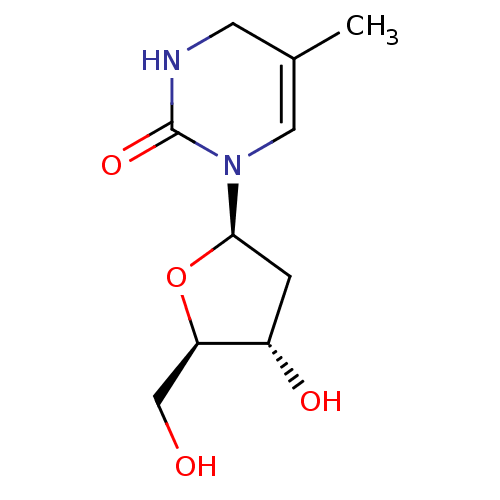
(CHEMBL3142412)Show SMILES CC1=CN([C@H]2C[C@H](O)[C@@H](CO)O2)C(=O)NC1 |r,t:1| Show InChI InChI=1S/C10H16N2O4/c1-6-3-11-10(15)12(4-6)9-2-7(14)8(5-13)16-9/h4,7-9,13-14H,2-3,5H2,1H3,(H,11,15)/t7-,8+,9+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
Bioorg Med Chem Lett 6: 2405-2410 (1996)
Article DOI: 10.1016/0960-894X(96)00444-1
BindingDB Entry DOI: 10.7270/Q2Z31ZMC |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50288058
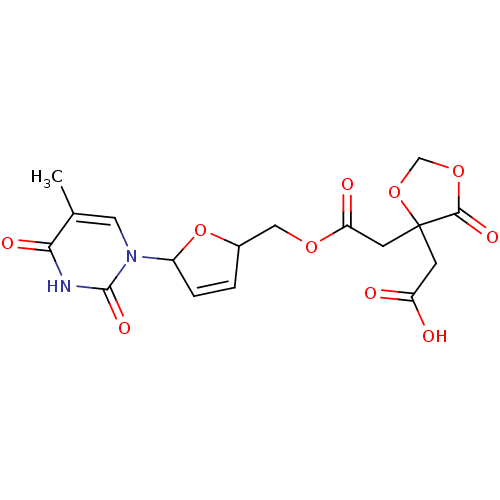
(CHEMBL78852 | {4-[5-(5-Methyl-2,4-dioxo-3,4-dihydr...)Show SMILES Cc1cn(C2OC(COC(=O)CC3(CC(O)=O)OCOC3=O)C=C2)c(=O)[nH]c1=O |c:23| Show InChI InChI=1S/C17H18N2O10/c1-9-6-19(16(25)18-14(9)23)11-3-2-10(29-11)7-26-13(22)5-17(4-12(20)21)15(24)27-8-28-17/h2-3,6,10-11H,4-5,7-8H2,1H3,(H,20,21)(H,18,23,25) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
Bioorg Med Chem Lett 6: 2405-2410 (1996)
Article DOI: 10.1016/0960-894X(96)00444-1
BindingDB Entry DOI: 10.7270/Q2Z31ZMC |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50288057
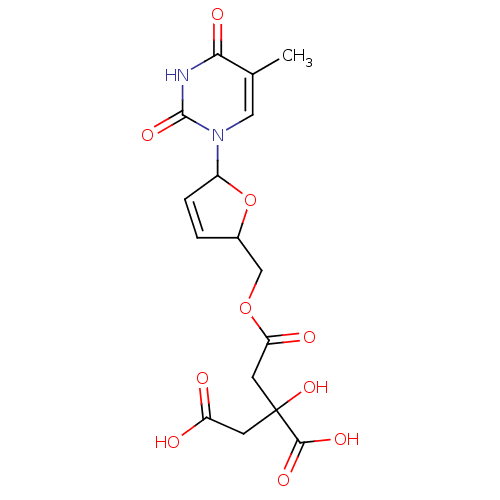
(3-Carboxy-3-hydroxy-pentanedioic acid mono-[5-(5-m...)Show SMILES Cc1cn(C2OC(COC(=O)CC(O)(CC(O)=O)C(O)=O)C=C2)c(=O)[nH]c1=O |c:21| Show InChI InChI=1S/C16H18N2O10/c1-8-6-18(15(25)17-13(8)22)10-3-2-9(28-10)7-27-12(21)5-16(26,14(23)24)4-11(19)20/h2-3,6,9-10,26H,4-5,7H2,1H3,(H,19,20)(H,23,24)(H,17,22,25) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
Bioorg Med Chem Lett 6: 2405-2410 (1996)
Article DOI: 10.1016/0960-894X(96)00444-1
BindingDB Entry DOI: 10.7270/Q2Z31ZMC |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50288063
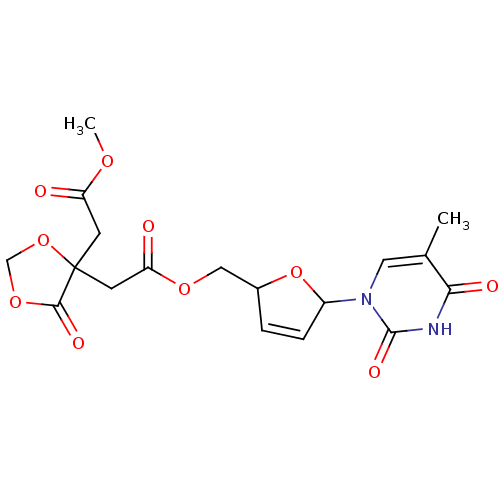
((4-Methoxycarbonylmethyl-5-oxo-[1,3]dioxolan-4-yl)...)Show SMILES COC(=O)CC1(CC(=O)OCC2OC(C=C2)n2cc(C)c(=O)[nH]c2=O)OCOC1=O |c:14| Show InChI InChI=1S/C18H20N2O10/c1-10-7-20(17(25)19-15(10)23)12-4-3-11(30-12)8-27-14(22)6-18(5-13(21)26-2)16(24)28-9-29-18/h3-4,7,11-12H,5-6,8-9H2,1-2H3,(H,19,23,25) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
Bioorg Med Chem Lett 6: 2405-2410 (1996)
Article DOI: 10.1016/0960-894X(96)00444-1
BindingDB Entry DOI: 10.7270/Q2Z31ZMC |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50002692

((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 reverse transcriptase. |
Bioorg Med Chem Lett 6: 2405-2410 (1996)
Article DOI: 10.1016/0960-894X(96)00444-1
BindingDB Entry DOI: 10.7270/Q2Z31ZMC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data