 Found 27 hits of Enzyme Inhibition Constant Data
Found 27 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neprilysin
(Homo sapiens (Human)) | BDBM50403595
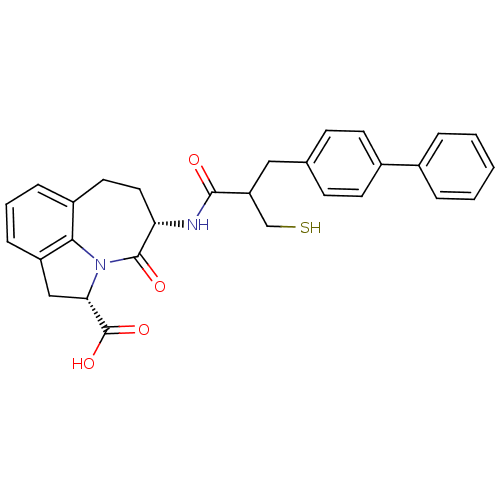
(CHEMBL2112402)Show SMILES OC(=O)[C@@H]1Cc2cccc3CC[C@H](NC(=O)C(CS)Cc4ccc(cc4)-c4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C29H28N2O4S/c32-27(23(17-36)15-18-9-11-20(12-10-18)19-5-2-1-3-6-19)30-24-14-13-21-7-4-8-22-16-25(29(34)35)31(26(21)22)28(24)33/h1-12,23-25,36H,13-17H2,(H,30,32)(H,34,35)/t23?,24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50403595

(CHEMBL2112402)Show SMILES OC(=O)[C@@H]1Cc2cccc3CC[C@H](NC(=O)C(CS)Cc4ccc(cc4)-c4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C29H28N2O4S/c32-27(23(17-36)15-18-9-11-20(12-10-18)19-5-2-1-3-6-19)30-24-14-13-21-7-4-8-22-16-25(29(34)35)31(26(21)22)28(24)33/h1-12,23-25,36H,13-17H2,(H,30,32)(H,34,35)/t23?,24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50288346
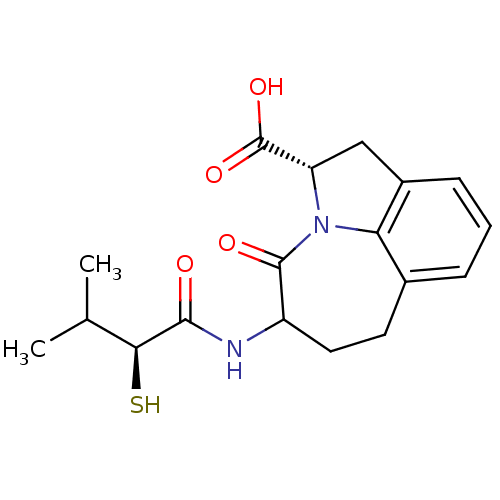
((S)-5-((S)-2-Mercapto-3-methyl-butyrylamino)-4-oxo...)Show SMILES CC(C)[C@H](S)C(=O)NC1CCc2cccc3C[C@H](N(c23)C1=O)C(O)=O Show InChI InChI=1S/C18H22N2O4S/c1-9(2)15(25)16(21)19-12-7-6-10-4-3-5-11-8-13(18(23)24)20(14(10)11)17(12)22/h3-5,9,12-13,15,25H,6-8H2,1-2H3,(H,19,21)(H,23,24)/t12?,13-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50403594
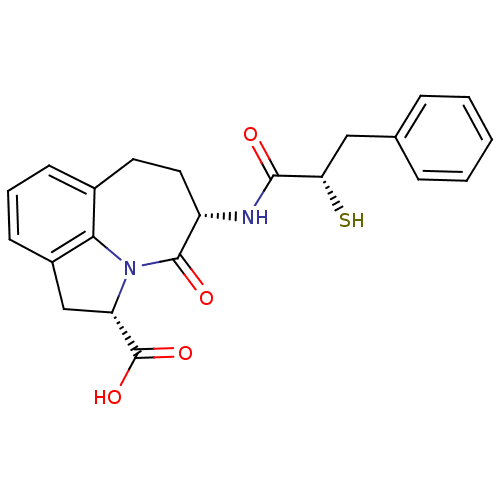
(CHEMBL2112403)Show SMILES OC(=O)[C@@H]1Cc2cccc3CC[C@H](NC(=O)[C@@H](S)Cc4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C22H22N2O4S/c25-20(18(29)11-13-5-2-1-3-6-13)23-16-10-9-14-7-4-8-15-12-17(22(27)28)24(19(14)15)21(16)26/h1-8,16-18,29H,9-12H2,(H,23,25)(H,27,28)/t16-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50288349
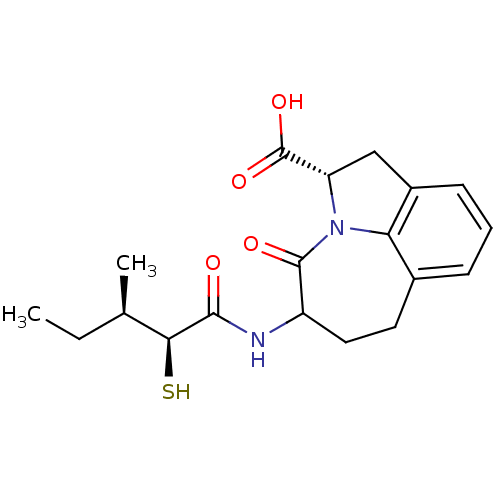
((S)-5-((2S,3R)-2-Mercapto-3-methyl-pentanoylamino)...)Show SMILES CC[C@@H](C)[C@H](S)C(=O)NC1CCc2cccc3C[C@H](N(c23)C1=O)C(O)=O Show InChI InChI=1S/C19H24N2O4S/c1-3-10(2)16(26)17(22)20-13-8-7-11-5-4-6-12-9-14(19(24)25)21(15(11)12)18(13)23/h4-6,10,13-14,16,26H,3,7-9H2,1-2H3,(H,20,22)(H,24,25)/t10-,13?,14+,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50403594

(CHEMBL2112403)Show SMILES OC(=O)[C@@H]1Cc2cccc3CC[C@H](NC(=O)[C@@H](S)Cc4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C22H22N2O4S/c25-20(18(29)11-13-5-2-1-3-6-13)23-16-10-9-14-7-4-8-15-12-17(22(27)28)24(19(14)15)21(16)26/h1-8,16-18,29H,9-12H2,(H,23,25)(H,27,28)/t16-,17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50403593

(CHEMBL2111596)Show SMILES OC(=O)[C@@H]1Cc2cccc3CC[C@@H](NC(=O)[C@@H](S)Cc4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C22H22N2O4S/c25-20(18(29)11-13-5-2-1-3-6-13)23-16-10-9-14-7-4-8-15-12-17(22(27)28)24(19(14)15)21(16)26/h1-8,16-18,29H,9-12H2,(H,23,25)(H,27,28)/t16-,17+,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50403595

(CHEMBL2112402)Show SMILES OC(=O)[C@@H]1Cc2cccc3CC[C@H](NC(=O)C(CS)Cc4ccc(cc4)-c4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C29H28N2O4S/c32-27(23(17-36)15-18-9-11-20(12-10-18)19-5-2-1-3-6-19)30-24-14-13-21-7-4-8-22-16-25(29(34)35)31(26(21)22)28(24)33/h1-12,23-25,36H,13-17H2,(H,30,32)(H,34,35)/t23?,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50403595

(CHEMBL2112402)Show SMILES OC(=O)[C@@H]1Cc2cccc3CC[C@H](NC(=O)C(CS)Cc4ccc(cc4)-c4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C29H28N2O4S/c32-27(23(17-36)15-18-9-11-20(12-10-18)19-5-2-1-3-6-19)30-24-14-13-21-7-4-8-22-16-25(29(34)35)31(26(21)22)28(24)33/h1-12,23-25,36H,13-17H2,(H,30,32)(H,34,35)/t23?,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50288353

((S)-5-((R)-2-Mercapto-3-phenyl-propionylamino)-4-o...)Show SMILES OC(=O)[C@@H]1Cc2cccc3CCC(NC(=O)[C@H](S)Cc4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C22H22N2O4S/c25-20(18(29)11-13-5-2-1-3-6-13)23-16-10-9-14-7-4-8-15-12-17(22(27)28)24(19(14)15)21(16)26/h1-8,16-18,29H,9-12H2,(H,23,25)(H,27,28)/t16?,17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50288354

((S)-5-((S)-3-Biphenyl-4-yl-2-mercapto-propionylami...)Show SMILES OC(=O)[C@@H]1Cc2cccc3CCC(NC(=O)[C@@H](S)Cc4ccc(cc4)-c4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C28H26N2O4S/c31-26(24(35)15-17-9-11-19(12-10-17)18-5-2-1-3-6-18)29-22-14-13-20-7-4-8-21-16-23(28(33)34)30(25(20)21)27(22)32/h1-12,22-24,35H,13-16H2,(H,29,31)(H,33,34)/t22?,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50288348

((S)-5-((S)-2-Cyclohexyl-2-mercapto-acetylamino)-4-...)Show SMILES OC(=O)[C@@H]1Cc2cccc3CCC(NC(=O)[C@@H](S)C4CCCCC4)C(=O)N1c23 Show InChI InChI=1S/C21H26N2O4S/c24-19(18(28)13-5-2-1-3-6-13)22-15-10-9-12-7-4-8-14-11-16(21(26)27)23(17(12)14)20(15)25/h4,7-8,13,15-16,18,28H,1-3,5-6,9-11H2,(H,22,24)(H,26,27)/t15?,16-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50288344

((S)-5-((S)-2-Mercapto-propionylamino)-4-oxo-1,2,4,...)Show SMILES C[C@H](S)C(=O)NC1CCc2cccc3C[C@H](N(c23)C1=O)C(O)=O Show InChI InChI=1S/C16H18N2O4S/c1-8(23)14(19)17-11-6-5-9-3-2-4-10-7-12(16(21)22)18(13(9)10)15(11)20/h2-4,8,11-12,23H,5-7H2,1H3,(H,17,19)(H,21,22)/t8-,11?,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50288352

((S)-5-[(1-Mercapto-cyclopentanecarbonyl)-amino]-4-...)Show SMILES OC(=O)[C@@H]1Cc2cccc3CCC(NC(=O)C4(S)CCCC4)C(=O)N1c23 Show InChI InChI=1S/C19H22N2O4S/c22-16-13(20-18(25)19(26)8-1-2-9-19)7-6-11-4-3-5-12-10-14(17(23)24)21(16)15(11)12/h3-5,13-14,26H,1-2,6-10H2,(H,20,25)(H,23,24)/t13?,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50288353

((S)-5-((R)-2-Mercapto-3-phenyl-propionylamino)-4-o...)Show SMILES OC(=O)[C@@H]1Cc2cccc3CCC(NC(=O)[C@H](S)Cc4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C22H22N2O4S/c25-20(18(29)11-13-5-2-1-3-6-13)23-16-10-9-14-7-4-8-15-12-17(22(27)28)24(19(14)15)21(16)26/h1-8,16-18,29H,9-12H2,(H,23,25)(H,27,28)/t16?,17-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 283 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50288351
((S)-5-[(2-Mercapto-indane-2-carbonyl)-amino]-4-oxo...)Show SMILES OC(=O)[C@@H]1Cc2cccc3CCC(NC(=O)C4(S)Cc5ccccc5C4)C(=O)N1c23 Show InChI InChI=1S/C23H22N2O4S/c26-20-17(24-22(29)23(30)11-15-4-1-2-5-16(15)12-23)9-8-13-6-3-7-14-10-18(21(27)28)25(20)19(13)14/h1-7,17-18,30H,8-12H2,(H,24,29)(H,27,28)/t17?,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 505 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50288346

((S)-5-((S)-2-Mercapto-3-methyl-butyrylamino)-4-oxo...)Show SMILES CC(C)[C@H](S)C(=O)NC1CCc2cccc3C[C@H](N(c23)C1=O)C(O)=O Show InChI InChI=1S/C18H22N2O4S/c1-9(2)15(25)16(21)19-12-7-6-10-4-3-5-11-8-13(18(23)24)20(14(10)11)17(12)22/h3-5,9,12-13,15,25H,6-8H2,1-2H3,(H,19,21)(H,23,24)/t12?,13-,15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50403593

(CHEMBL2111596)Show SMILES OC(=O)[C@@H]1Cc2cccc3CC[C@@H](NC(=O)[C@@H](S)Cc4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C22H22N2O4S/c25-20(18(29)11-13-5-2-1-3-6-13)23-16-10-9-14-7-4-8-15-12-17(22(27)28)24(19(14)15)21(16)26/h1-8,16-18,29H,9-12H2,(H,23,25)(H,27,28)/t16-,17+,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50288348

((S)-5-((S)-2-Cyclohexyl-2-mercapto-acetylamino)-4-...)Show SMILES OC(=O)[C@@H]1Cc2cccc3CCC(NC(=O)[C@@H](S)C4CCCCC4)C(=O)N1c23 Show InChI InChI=1S/C21H26N2O4S/c24-19(18(28)13-5-2-1-3-6-13)22-15-10-9-12-7-4-8-14-11-16(21(26)27)23(17(12)14)20(15)25/h4,7-8,13,15-16,18,28H,1-3,5-6,9-11H2,(H,22,24)(H,26,27)/t15?,16-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50288344

((S)-5-((S)-2-Mercapto-propionylamino)-4-oxo-1,2,4,...)Show SMILES C[C@H](S)C(=O)NC1CCc2cccc3C[C@H](N(c23)C1=O)C(O)=O Show InChI InChI=1S/C16H18N2O4S/c1-8(23)14(19)17-11-6-5-9-3-2-4-10-7-12(16(21)22)18(13(9)10)15(11)20/h2-4,8,11-12,23H,5-7H2,1H3,(H,17,19)(H,21,22)/t8-,11?,12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50288354

((S)-5-((S)-3-Biphenyl-4-yl-2-mercapto-propionylami...)Show SMILES OC(=O)[C@@H]1Cc2cccc3CCC(NC(=O)[C@@H](S)Cc4ccc(cc4)-c4ccccc4)C(=O)N1c23 Show InChI InChI=1S/C28H26N2O4S/c31-26(24(35)15-17-9-11-19(12-10-17)18-5-2-1-3-6-18)29-22-14-13-20-7-4-8-21-16-23(28(33)34)30(25(20)21)27(22)32/h1-12,22-24,35H,13-16H2,(H,29,31)(H,33,34)/t22?,23-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50288349

((S)-5-((2S,3R)-2-Mercapto-3-methyl-pentanoylamino)...)Show SMILES CC[C@@H](C)[C@H](S)C(=O)NC1CCc2cccc3C[C@H](N(c23)C1=O)C(O)=O Show InChI InChI=1S/C19H24N2O4S/c1-3-10(2)16(26)17(22)20-13-8-7-11-5-4-6-12-9-14(19(24)25)21(15(11)12)18(13)23/h4-6,10,13-14,16,26H,3,7-9H2,1-2H3,(H,20,22)(H,24,25)/t10-,13?,14+,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50288350
((S)-5-(2-Mercapto-2-methyl-propionylamino)-4-oxo-1...)Show SMILES CC(C)(S)C(=O)NC1CCc2cccc3C[C@H](N(c23)C1=O)C(O)=O Show InChI InChI=1S/C17H20N2O4S/c1-17(2,24)16(23)18-11-7-6-9-4-3-5-10-8-12(15(21)22)19(13(9)10)14(11)20/h3-5,11-12,24H,6-8H2,1-2H3,(H,18,23)(H,21,22)/t11?,12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50288355

((S)-5-(2-Mercapto-acetylamino)-4-oxo-1,2,4,5,6,7-h...)Show SMILES OC(=O)[C@@H]1Cc2cccc3CCC(NC(=O)CS)C(=O)N1c23 Show InChI InChI=1S/C15H16N2O4S/c18-12(7-22)16-10-5-4-8-2-1-3-9-6-11(15(20)21)17(13(8)9)14(10)19/h1-3,10-11,22H,4-7H2,(H,16,18)(H,20,21)/t10?,11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50288350

((S)-5-(2-Mercapto-2-methyl-propionylamino)-4-oxo-1...)Show SMILES CC(C)(S)C(=O)NC1CCc2cccc3C[C@H](N(c23)C1=O)C(O)=O Show InChI InChI=1S/C17H20N2O4S/c1-17(2,24)16(23)18-11-7-6-9-4-3-5-10-8-12(15(21)22)19(13(9)10)14(11)20/h3-5,11-12,24H,6-8H2,1-2H3,(H,18,23)(H,21,22)/t11?,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50288351

((S)-5-[(2-Mercapto-indane-2-carbonyl)-amino]-4-oxo...)Show SMILES OC(=O)[C@@H]1Cc2cccc3CCC(NC(=O)C4(S)Cc5ccccc5C4)C(=O)N1c23 Show InChI InChI=1S/C23H22N2O4S/c26-20-17(24-22(29)23(30)11-15-4-1-2-5-16(15)12-23)9-8-13-6-3-7-14-10-18(21(27)28)25(20)19(13)14/h1-7,17-18,30H,8-12H2,(H,24,29)(H,27,28)/t17?,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Angiotensin I converting enzyme |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50288352

((S)-5-[(1-Mercapto-cyclopentanecarbonyl)-amino]-4-...)Show SMILES OC(=O)[C@@H]1Cc2cccc3CCC(NC(=O)C4(S)CCCC4)C(=O)N1c23 Show InChI InChI=1S/C19H22N2O4S/c22-16-13(20-18(25)19(26)8-1-2-9-19)7-6-11-4-3-5-12-10-14(17(23)24)21(16)15(11)12/h3-5,13-14,26H,1-2,6-10H2,(H,20,25)(H,23,24)/t13?,14-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of neutral endopeptidase |
Bioorg Med Chem Lett 6: 2875-2880 (1996)
Article DOI: 10.1016/S0960-894X(96)00529-X
BindingDB Entry DOI: 10.7270/Q27D2V3C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data