

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50299749 ((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM18358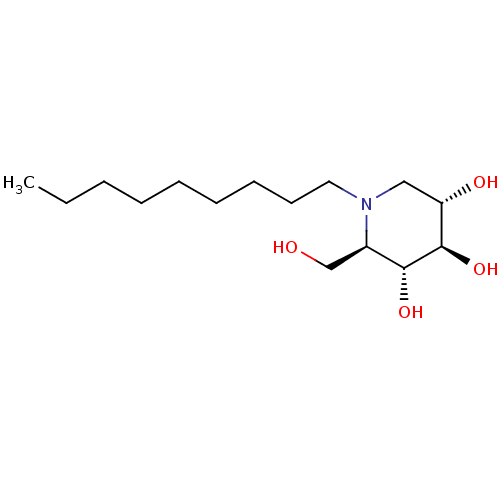 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335390 (CHEMBL1651630 | N-Hexoxypentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335388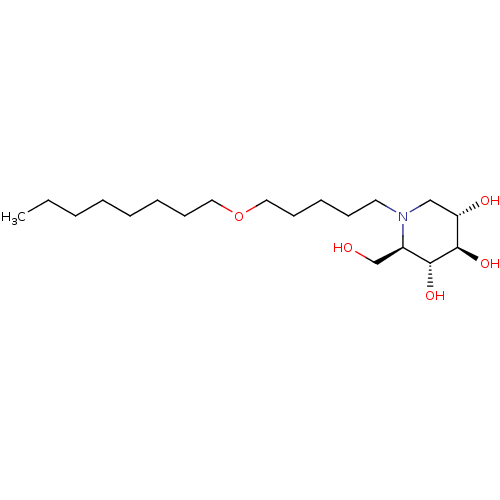 (CHEMBL1651632 | N-Octoxypentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335393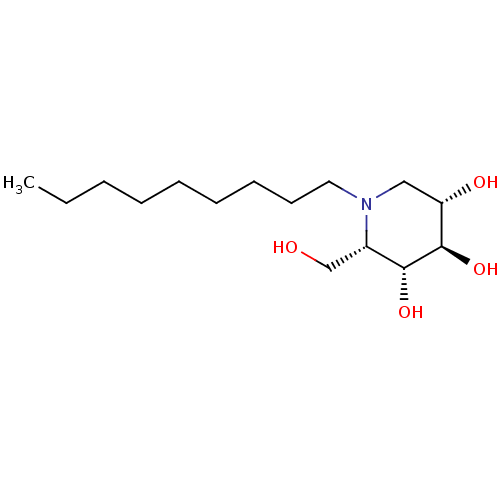 (CHEMBL1651627 | N-Nonyl-L-ido-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335382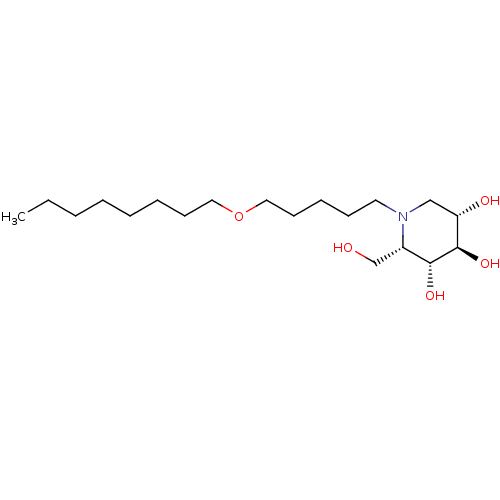 (CHEMBL1651638 | N-Octoxypentyl-L-ido-1-deoxynojiri...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335385 (CHEMBL1651635 | N-Pentoxypentyl-L-ido-1-deoxynojir...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335383 (CHEMBL1651637 | N-Heptoxypentyl-L-ido-1-deoxynojir...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335389 (CHEMBL1651631 | N-Heptoxypentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM18357 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-octylpiperidine-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335394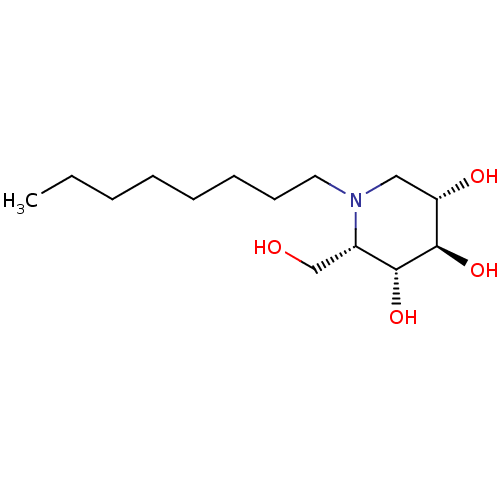 (CHEMBL1651626 | N-Octyl-L-ido-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335384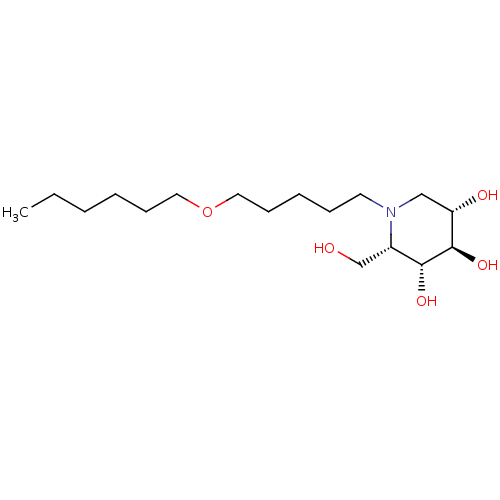 (CHEMBL1651636 | N-Hexoxypentyl-L-ido-1-deoxynojiri...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50312527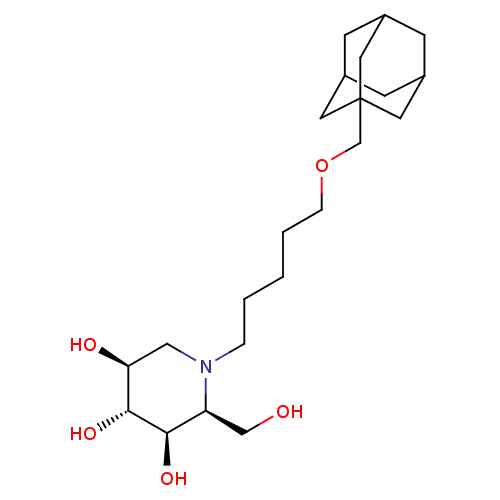 (CHEMBL1086996 | N-[5-(Adamantan-1-yl-methoxy)-pent...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335387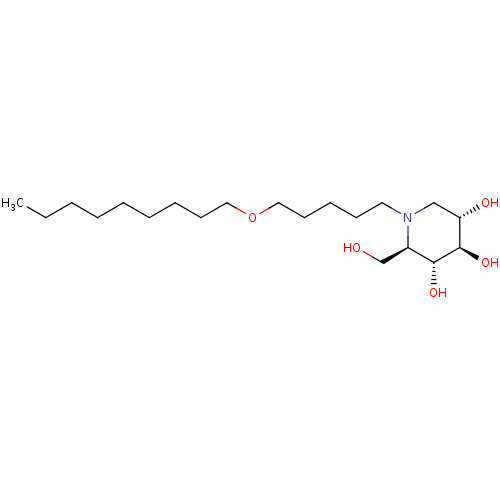 (CHEMBL1651633 | N-Nonoxypentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335395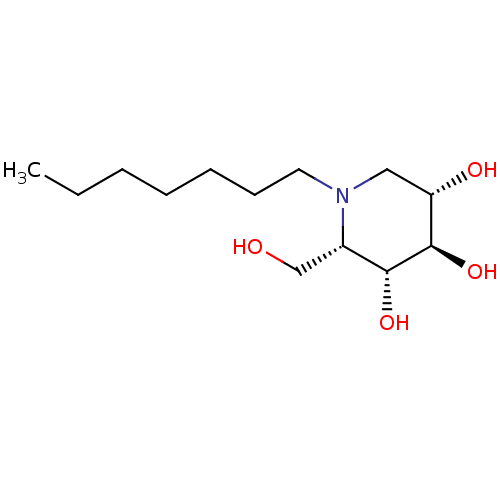 (CHEMBL1651555 | N-Heptyl-L-ido-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335391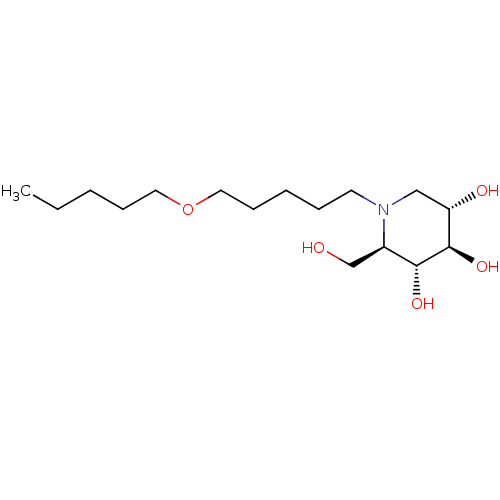 (CHEMBL1651629 | N-Pentoxypentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335398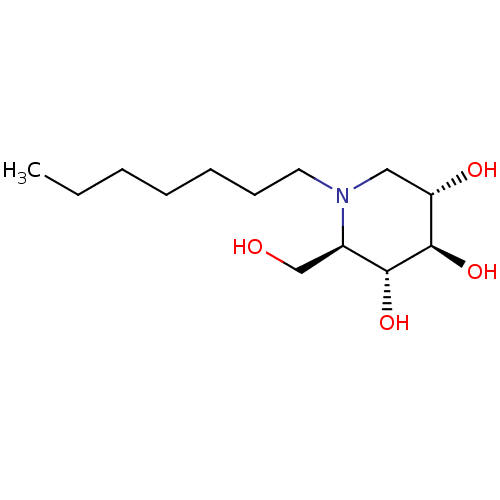 (CHEMBL1651551 | N-Heptyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335381 (CHEMBL1651639 | N-Nonoxypentyl-L-ido-1-deoxynojiri...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335383 (CHEMBL1651637 | N-Heptoxypentyl-L-ido-1-deoxynojir...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335382 (CHEMBL1651638 | N-Octoxypentyl-L-ido-1-deoxynojiri...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335392 (CHEMBL1651628 | N-Butoxypentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335386 (CHEMBL1651634 | N-Butoxypentyl-L-ido-1-deoxynojiri...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335387 (CHEMBL1651633 | N-Nonoxypentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50312527 (CHEMBL1086996 | N-[5-(Adamantan-1-yl-methoxy)-pent...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335384 (CHEMBL1651636 | N-Hexoxypentyl-L-ido-1-deoxynojiri...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM18356 ((2R,3R,4R,5S)-1-hexyl-2-(hydroxymethyl)piperidine-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335396 (CHEMBL1651554 | N-Hexyl-L-ido-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335385 (CHEMBL1651635 | N-Pentoxypentyl-L-ido-1-deoxynojir...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50299749 ((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50299749 ((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335388 (CHEMBL1651632 | N-Octoxypentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335397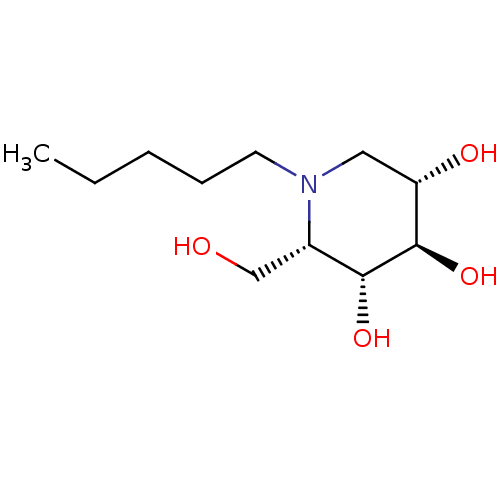 (CHEMBL1651553 | N-Pentyl-L-ido-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335389 (CHEMBL1651631 | N-Heptoxypentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM18358 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50335399 (CHEMBL1651549 | N-Pentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335388 (CHEMBL1651632 | N-Octoxypentyl-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50299749 ((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335387 (CHEMBL1651633 | N-Nonoxypentyl-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335398 (CHEMBL1651551 | N-Heptyl-1-deoxynojirimycin) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM18357 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-octylpiperidine-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335390 (CHEMBL1651630 | N-Hexoxypentyl-1-deoxynojirimycin) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50312526 (CHEMBL1076754 | N-Butyl-L-ido-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA2 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335390 (CHEMBL1651630 | N-Hexoxypentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335389 (CHEMBL1651631 | N-Heptoxypentyl-1-deoxynojirimycin) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335388 (CHEMBL1651632 | N-Octoxypentyl-1-deoxynojirimycin) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM18356 ((2R,3R,4R,5S)-1-hexyl-2-(hydroxymethyl)piperidine-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335391 (CHEMBL1651629 | N-Pentoxypentyl-1-deoxynojirimycin) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18358 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335389 (CHEMBL1651631 | N-Heptoxypentyl-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335393 (CHEMBL1651627 | N-Nonyl-L-ido-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50312527 (CHEMBL1086996 | N-[5-(Adamantan-1-yl-methoxy)-pent...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335392 (CHEMBL1651628 | N-Butoxypentyl-1-deoxynojirimycin) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335386 (CHEMBL1651634 | N-Butoxypentyl-L-ido-1-deoxynojiri...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335391 (CHEMBL1651629 | N-Pentoxypentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335399 (CHEMBL1651549 | N-Pentyl-1-deoxynojirimycin) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335387 (CHEMBL1651633 | N-Nonoxypentyl-1-deoxynojirimycin) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50299749 ((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM18358 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335392 (CHEMBL1651628 | N-Butoxypentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM18357 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-octylpiperidine-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335391 (CHEMBL1651629 | N-Pentoxypentyl-1-deoxynojirimycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18357 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-octylpiperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335394 (CHEMBL1651626 | N-Octyl-L-ido-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335395 (CHEMBL1651555 | N-Heptyl-L-ido-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335390 (CHEMBL1651630 | N-Hexoxypentyl-1-deoxynojirimycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335389 (CHEMBL1651631 | N-Heptoxypentyl-1-deoxynojirimycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM18358 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335392 (CHEMBL1651628 | N-Butoxypentyl-1-deoxynojirimycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM18357 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-octylpiperidine-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335398 (CHEMBL1651551 | N-Heptyl-1-deoxynojirimycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM18356 ((2R,3R,4R,5S)-1-hexyl-2-(hydroxymethyl)piperidine-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335399 (CHEMBL1651549 | N-Pentyl-1-deoxynojirimycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335388 (CHEMBL1651632 | N-Octoxypentyl-1-deoxynojirimycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335381 (CHEMBL1651639 | N-Nonoxypentyl-L-ido-1-deoxynojiri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335397 (CHEMBL1651553 | N-Pentyl-L-ido-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335382 (CHEMBL1651638 | N-Octoxypentyl-L-ido-1-deoxynojiri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335398 (CHEMBL1651551 | N-Heptyl-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50312526 (CHEMBL1076754 | N-Butyl-L-ido-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335399 (CHEMBL1651549 | N-Pentyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM18356 ((2R,3R,4R,5S)-1-hexyl-2-(hydroxymethyl)piperidine-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335387 (CHEMBL1651633 | N-Nonoxypentyl-1-deoxynojirimycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335394 (CHEMBL1651626 | N-Octyl-L-ido-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335392 (CHEMBL1651628 | N-Butoxypentyl-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50299749 ((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335383 (CHEMBL1651637 | N-Heptoxypentyl-L-ido-1-deoxynojir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335398 (CHEMBL1651551 | N-Heptyl-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50335396 (CHEMBL1651554 | N-Hexyl-L-ido-1-deoxynojirimycin) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335393 (CHEMBL1651627 | N-Nonyl-L-ido-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335394 (CHEMBL1651626 | N-Octyl-L-ido-1-deoxynojirimycin) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18356 ((2R,3R,4R,5S)-1-hexyl-2-(hydroxymethyl)piperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335384 (CHEMBL1651636 | N-Hexoxypentyl-L-ido-1-deoxynojiri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335385 (CHEMBL1651635 | N-Pentoxypentyl-L-ido-1-deoxynojir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335394 (CHEMBL1651626 | N-Octyl-L-ido-1-deoxynojirimycin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM18358 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335385 (CHEMBL1651635 | N-Pentoxypentyl-L-ido-1-deoxynojir...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335390 (CHEMBL1651630 | N-Hexoxypentyl-1-deoxynojirimycin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335384 (CHEMBL1651636 | N-Hexoxypentyl-L-ido-1-deoxynojiri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335395 (CHEMBL1651555 | N-Heptyl-L-ido-1-deoxynojirimycin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335388 (CHEMBL1651632 | N-Octoxypentyl-1-deoxynojirimycin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335390 (CHEMBL1651630 | N-Hexoxypentyl-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335389 (CHEMBL1651631 | N-Heptoxypentyl-1-deoxynojirimycin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335383 (CHEMBL1651637 | N-Heptoxypentyl-L-ido-1-deoxynojir...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335391 (CHEMBL1651629 | N-Pentoxypentyl-1-deoxynojirimycin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM18357 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-octylpiperidine-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335392 (CHEMBL1651628 | N-Butoxypentyl-1-deoxynojirimycin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335382 (CHEMBL1651638 | N-Octoxypentyl-L-ido-1-deoxynojiri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335381 (CHEMBL1651639 | N-Nonoxypentyl-L-ido-1-deoxynojiri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335387 (CHEMBL1651633 | N-Nonoxypentyl-1-deoxynojirimycin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335386 (CHEMBL1651634 | N-Butoxypentyl-L-ido-1-deoxynojiri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335398 (CHEMBL1651551 | N-Heptyl-1-deoxynojirimycin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335399 (CHEMBL1651549 | N-Pentyl-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335396 (CHEMBL1651554 | N-Hexyl-L-ido-1-deoxynojirimycin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335394 (CHEMBL1651626 | N-Octyl-L-ido-1-deoxynojirimycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335396 (CHEMBL1651554 | N-Hexyl-L-ido-1-deoxynojirimycin) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335395 (CHEMBL1651555 | N-Heptyl-L-ido-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM18356 ((2R,3R,4R,5S)-1-hexyl-2-(hydroxymethyl)piperidine-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335391 (CHEMBL1651629 | N-Pentoxypentyl-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50312526 (CHEMBL1076754 | N-Butyl-L-ido-1-deoxynojirimycin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50312527 (CHEMBL1086996 | N-[5-(Adamantan-1-yl-methoxy)-pent...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335382 (CHEMBL1651638 | N-Octoxypentyl-L-ido-1-deoxynojiri...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335395 (CHEMBL1651555 | N-Heptyl-L-ido-1-deoxynojirimycin) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335382 (CHEMBL1651638 | N-Octoxypentyl-L-ido-1-deoxynojiri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335396 (CHEMBL1651554 | N-Hexyl-L-ido-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335397 (CHEMBL1651553 | N-Pentyl-L-ido-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335395 (CHEMBL1651555 | N-Heptyl-L-ido-1-deoxynojirimycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335399 (CHEMBL1651549 | N-Pentyl-1-deoxynojirimycin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335383 (CHEMBL1651637 | N-Heptoxypentyl-L-ido-1-deoxynojir...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335381 (CHEMBL1651639 | N-Nonoxypentyl-L-ido-1-deoxynojiri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335383 (CHEMBL1651637 | N-Heptoxypentyl-L-ido-1-deoxynojir...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50312526 (CHEMBL1076754 | N-Butyl-L-ido-1-deoxynojirimycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335384 (CHEMBL1651636 | N-Hexoxypentyl-L-ido-1-deoxynojiri...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335393 (CHEMBL1651627 | N-Nonyl-L-ido-1-deoxynojirimycin) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335386 (CHEMBL1651634 | N-Butoxypentyl-L-ido-1-deoxynojiri...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50312526 (CHEMBL1076754 | N-Butyl-L-ido-1-deoxynojirimycin) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50335386 (CHEMBL1651634 | N-Butoxypentyl-L-ido-1-deoxynojiri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50312527 (CHEMBL1086996 | N-[5-(Adamantan-1-yl-methoxy)-pent...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335393 (CHEMBL1651627 | N-Nonyl-L-ido-1-deoxynojirimycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335396 (CHEMBL1651554 | N-Hexyl-L-ido-1-deoxynojirimycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335393 (CHEMBL1651627 | N-Nonyl-L-ido-1-deoxynojirimycin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335385 (CHEMBL1651635 | N-Pentoxypentyl-L-ido-1-deoxynojir...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335397 (CHEMBL1651553 | N-Pentyl-L-ido-1-deoxynojirimycin) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335385 (CHEMBL1651635 | N-Pentoxypentyl-L-ido-1-deoxynojir...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335397 (CHEMBL1651553 | N-Pentyl-L-ido-1-deoxynojirimycin) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase (Mus musculus) | BDBM50335381 (CHEMBL1651639 | N-Nonoxypentyl-L-ido-1-deoxynojiri...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of sucrase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50312526 (CHEMBL1076754 | N-Butyl-L-ido-1-deoxynojirimycin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant GBA1 preincuabated with compound for 30 mins using 4-methylumbelliferyl-B-glucoside substrate by fluorimetric assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335384 (CHEMBL1651636 | N-Hexoxypentyl-L-ido-1-deoxynojiri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50312527 (CHEMBL1086996 | N-[5-(Adamantan-1-yl-methoxy)-pent...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Mus musculus (Mouse)) | BDBM50335386 (CHEMBL1651634 | N-Butoxypentyl-L-ido-1-deoxynojiri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of maltase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Mus musculus) | BDBM50335397 (CHEMBL1651553 | N-Pentyl-L-ido-1-deoxynojirimycin) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of lactase in mouse intestinal input by glucose release assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||