

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP)prepared from microsomal fractions of rat small intestine | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282586 (3-(S)-1H-Indol-3-yl-2-[(S)-4-methyl-2-(phosphonome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP)prepared from microsomal fractions of rat small intestine | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282588 (3-(S)-1H-Indol-3-yl-2-[(S)-4-methyl-2-(3-phenyl-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP)prepared from microsomal fractions of rat small intestine | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282589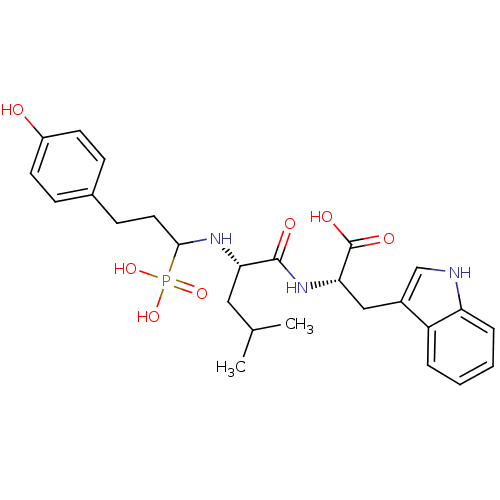 (2-{(S)-2-[3-(4-Hydroxy-phenyl)-1-phosphono-propyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP)prepared from microsomal fractions of rat small intestine | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282593 (3-(S)-1H-Indol-3-yl-2-[(S)-4-methyl-2-(3-naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP)prepared from microsomal fractions of rat small intestine | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Bos taurus) | BDBM50282593 (3-(S)-1H-Indol-3-yl-2-[(S)-4-methyl-2-(3-naphthale...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Endothelin-converting enzyme (ECE) from microsomal fractions of bovine cultured endothelial cells | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282592 (3-(S)-1H-Indol-3-yl-2-[(S)-4-methyl-2-(1-phosphono...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP)prepared from microsomal fractions of rat small intestine | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282587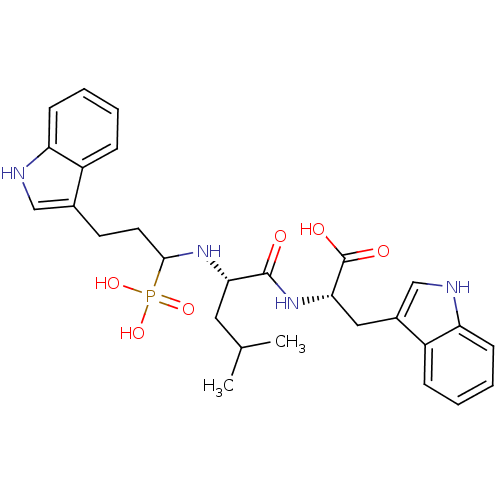 (3-(S)-1H-Indol-3-yl-2-{(S)-2-[3-(1H-indol-3-yl)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP)prepared from microsomal fractions of rat small intestine | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Bos taurus) | BDBM50282595 (CHEMBL281562 | PO3 2-Leu-Trp-O-3K) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Endothelin-converting enzyme (ECE) from microsomal fractions of bovine cultured endothelial cells | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50282590 (3-(S)-1H-Indol-3-yl-2-[(S)-4-methyl-2-(4-phenyl-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against neutral endopeptidase (NEP)prepared from microsomal fractions of rat small intestine | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Bos taurus) | BDBM50282588 (3-(S)-1H-Indol-3-yl-2-[(S)-4-methyl-2-(3-phenyl-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Endothelin-converting enzyme (ECE) from microsomal fractions of bovine cultured endothelial cells | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Bos taurus) | BDBM50282585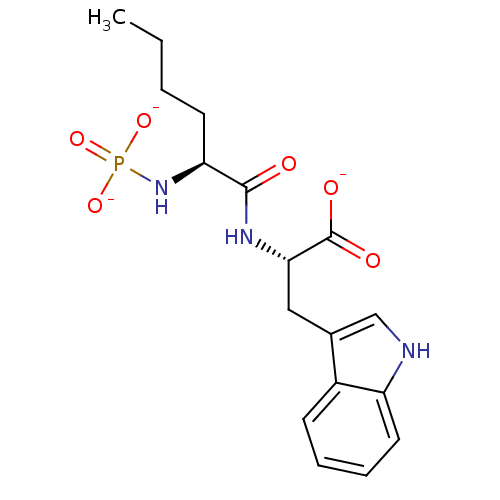 (CHEMBL22509 | PO3 2-Nle-Trp-O-3K) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Endothelin-converting enzyme (ECE) from microsomal fractions of bovine cultured endothelial cells | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Bos taurus) | BDBM50282587 (3-(S)-1H-Indol-3-yl-2-{(S)-2-[3-(1H-indol-3-yl)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Endothelin-converting enzyme (ECE) from microsomal fractions of bovine cultured endothelial cells | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Bos taurus) | BDBM50282591 (CHEMBL281785 | PO3 2-Ile-Trp-O-3K) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Endothelin-converting enzyme (ECE) from microsomal fractions of bovine cultured endothelial cells | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Bos taurus) | BDBM50282589 (2-{(S)-2-[3-(4-Hydroxy-phenyl)-1-phosphono-propyla...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Endothelin-converting enzyme (ECE) from microsomal fractions of bovine cultured endothelial cells | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Bos taurus) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Endothelin-converting enzyme (ECE) from microsomal fractions of bovine cultured endothelial cells | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-converting enzyme 1 (Bos taurus) | BDBM50282586 (3-(S)-1H-Indol-3-yl-2-[(S)-4-methyl-2-(phosphonome...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Endothelin-converting enzyme (ECE) from microsomal fractions of bovine cultured endothelial cells | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Bos taurus) | BDBM50282590 (3-(S)-1H-Indol-3-yl-2-[(S)-4-methyl-2-(4-phenyl-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Endothelin-converting enzyme (ECE) from microsomal fractions of bovine cultured endothelial cells | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Bos taurus) | BDBM50282594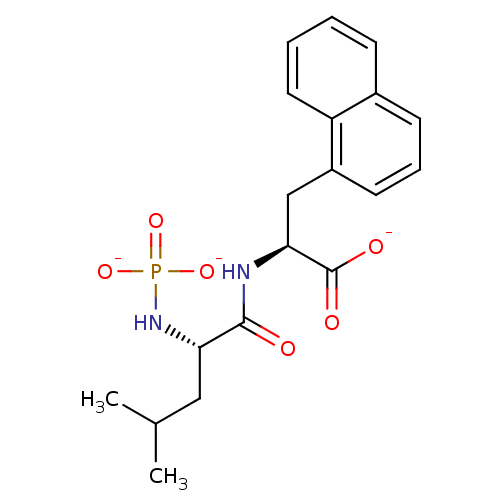 (CHEMBL280917 | PO3 2-Leu-Nal-O-3K) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Endothelin-converting enzyme (ECE) from microsomal fractions of bovine cultured endothelial cells | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Bos taurus) | BDBM50282592 (3-(S)-1H-Indol-3-yl-2-[(S)-4-methyl-2-(1-phosphono...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Endothelin-converting enzyme (ECE) from microsomal fractions of bovine cultured endothelial cells | Bioorg Med Chem Lett 4: 1257-1262 (1994) Article DOI: 10.1016/S0960-894X(01)80341-3 BindingDB Entry DOI: 10.7270/Q2KS6S2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||