 Found 32 hits of Enzyme Inhibition Constant Data
Found 32 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50342601

(CHEMBL1255901 | Huperzine A)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@]1(N)CC(C)=C2 |r,c:18,TLB:1:2:11.5.4:17.14.15| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10972
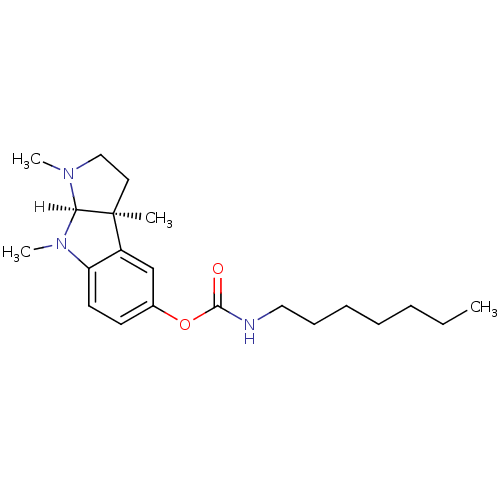
((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)NCCCCCCC)ccc1N2C Show InChI InChI=1S/C21H33N3O2/c1-5-6-7-8-9-13-22-20(25)26-16-10-11-18-17(15-16)21(2)12-14-23(3)19(21)24(18)4/h10-11,15,19H,5-9,12-14H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50342601

(CHEMBL1255901 | Huperzine A)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@]1(N)CC(C)=C2 |r,c:18,TLB:1:2:11.5.4:17.14.15| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50289094
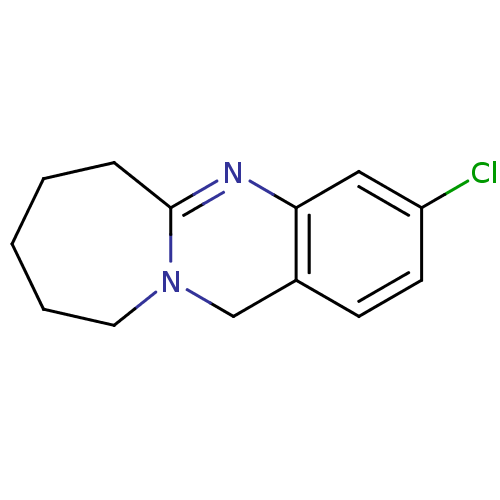
(3-Chloro-6,7,8,9,10,12-hexahydro-azepino[2,1-b]qui...)Show InChI InChI=1S/C13H15ClN2/c14-11-6-5-10-9-16-7-3-1-2-4-13(16)15-12(10)8-11/h5-6,8H,1-4,7,9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50289104
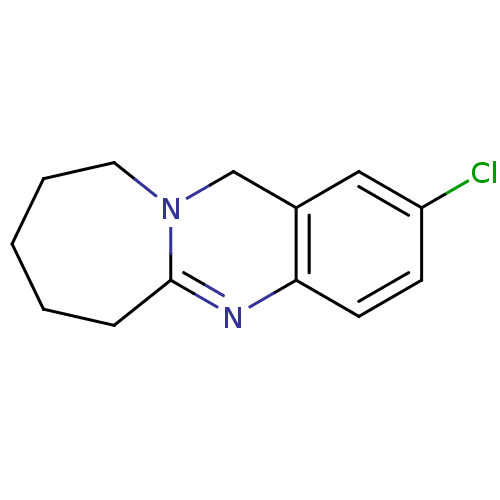
(2-Chloro-6,7,8,9,10,12-hexahydro-azepino[2,1-b]qui...)Show InChI InChI=1S/C13H15ClN2/c14-11-5-6-12-10(8-11)9-16-7-3-1-2-4-13(16)15-12/h5-6,8H,1-4,7,9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM10972

((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...)Show SMILES [H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)NCCCCCCC)ccc1N2C Show InChI InChI=1S/C21H33N3O2/c1-5-6-7-8-9-13-22-20(25)26-16-10-11-18-17(15-16)21(2)12-14-23(3)19(21)24(18)4/h10-11,15,19H,5-9,12-14H2,1-4H3,(H,22,25)/t19-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50289096
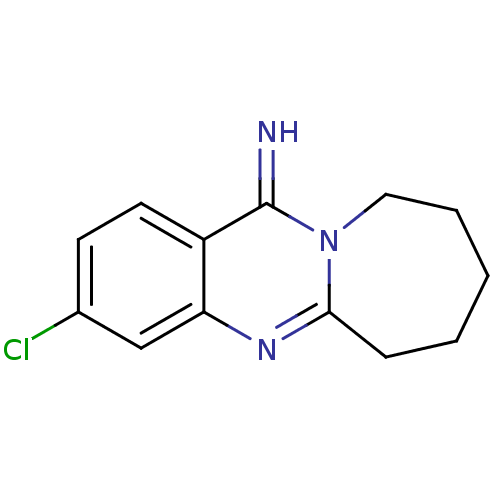
(3-Chloro-7,8,9,10-tetrahydro-6H-azepino[2,1-b]quin...)Show InChI InChI=1S/C13H14ClN3/c14-9-5-6-10-11(8-9)16-12-4-2-1-3-7-17(12)13(10)15/h5-6,8,15H,1-4,7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50289103
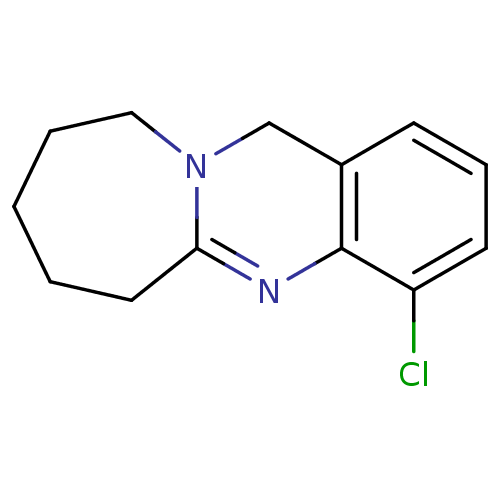
(4-Chloro-6,7,8,9,10,12-hexahydro-azepino[2,1-b]qui...)Show InChI InChI=1S/C13H15ClN2/c14-11-6-4-5-10-9-16-8-3-1-2-7-12(16)15-13(10)11/h4-6H,1-3,7-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50289099
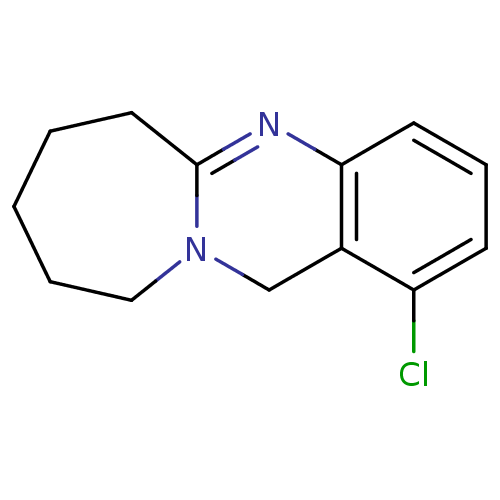
(1-Chloro-6,7,8,9,10,12-hexahydro-azepino[2,1-b]qui...)Show InChI InChI=1S/C13H15ClN2/c14-11-5-4-6-12-10(11)9-16-8-3-1-2-7-13(16)15-12/h4-6H,1-3,7-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50289094

(3-Chloro-6,7,8,9,10,12-hexahydro-azepino[2,1-b]qui...)Show InChI InChI=1S/C13H15ClN2/c14-11-6-5-10-9-16-7-3-1-2-4-13(16)15-12(10)8-11/h5-6,8H,1-4,7,9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50289095
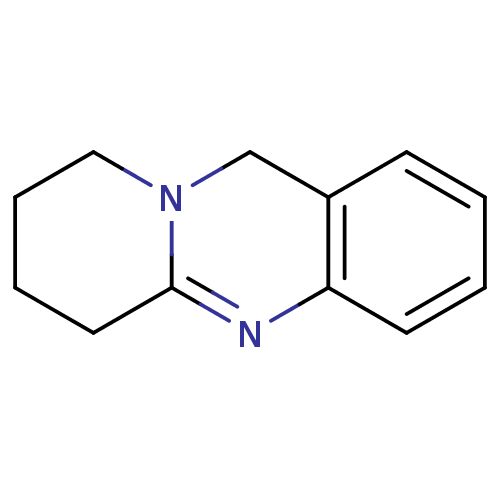
(6,8,9,11-Tetrahydro-7H-pyrido[2,1-b]quinazoline | ...)Show InChI InChI=1S/C12H14N2/c1-2-6-11-10(5-1)9-14-8-4-3-7-12(14)13-11/h1-2,5-6H,3-4,7-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50289102

(1,2,3,9-Tetrahydro-pyrrolo[2,1-b]quinazoline | CHE...)Show InChI InChI=1S/C11H12N2/c1-2-5-10-9(4-1)8-13-7-3-6-11(13)12-10/h1-2,4-5H,3,6-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50289105
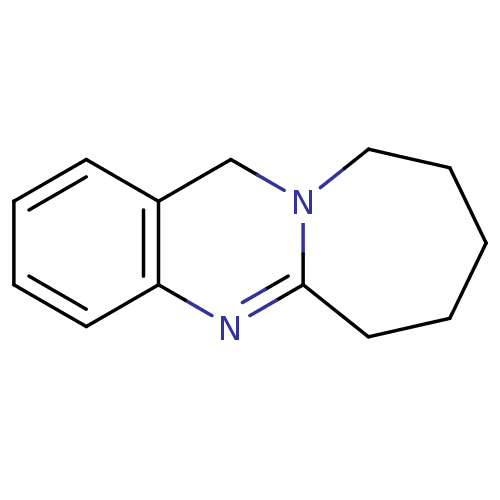
(6,7,8,9,10,12-Hexahydro-azepino[2,1-b]quinazoline ...)Show InChI InChI=1S/C13H16N2/c1-2-8-13-14-12-7-4-3-6-11(12)10-15(13)9-5-1/h3-4,6-7H,1-2,5,8-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50289099

(1-Chloro-6,7,8,9,10,12-hexahydro-azepino[2,1-b]qui...)Show InChI InChI=1S/C13H15ClN2/c14-11-5-4-6-12-10(11)9-16-8-3-1-2-7-13(16)15-12/h4-6H,1-3,7-9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50289098
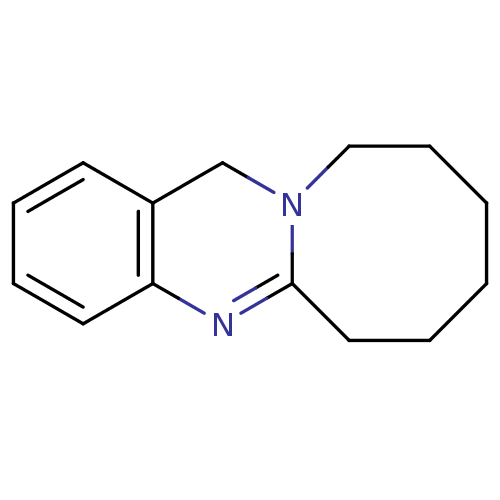
(6,8,9,10,11,12-Hexahydro-7H-5,11a-diaza-cycloocta[...)Show InChI InChI=1S/C14H18N2/c1-2-6-10-16-11-12-7-4-5-8-13(12)15-14(16)9-3-1/h4-5,7-8H,1-3,6,9-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50289104

(2-Chloro-6,7,8,9,10,12-hexahydro-azepino[2,1-b]qui...)Show InChI InChI=1S/C13H15ClN2/c14-11-5-6-12-10(8-11)9-16-7-3-1-2-4-13(16)15-12/h5-6,8H,1-4,7,9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50289097
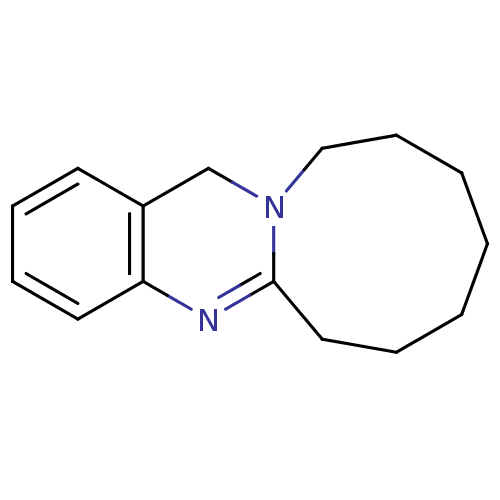
(6,7,8,9,10,11,12,13-Octahydro-5,12a-diaza-cyclonon...)Show InChI InChI=1S/C15H20N2/c1-2-4-10-15-16-14-9-6-5-8-13(14)12-17(15)11-7-3-1/h5-6,8-9H,1-4,7,10-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50289105

(6,7,8,9,10,12-Hexahydro-azepino[2,1-b]quinazoline ...)Show InChI InChI=1S/C13H16N2/c1-2-8-13-14-12-7-4-3-6-11(12)10-15(13)9-5-1/h3-4,6-7H,1-2,5,8-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50289103

(4-Chloro-6,7,8,9,10,12-hexahydro-azepino[2,1-b]qui...)Show InChI InChI=1S/C13H15ClN2/c14-11-6-4-5-10-9-16-8-3-1-2-7-12(16)15-13(10)11/h4-6H,1-3,7-9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50289102

(1,2,3,9-Tetrahydro-pyrrolo[2,1-b]quinazoline | CHE...)Show InChI InChI=1S/C11H12N2/c1-2-5-10-9(4-1)8-13-7-3-6-11(13)12-10/h1-2,4-5H,3,6-8H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50289096

(3-Chloro-7,8,9,10-tetrahydro-6H-azepino[2,1-b]quin...)Show InChI InChI=1S/C13H14ClN3/c14-9-5-6-10-11(8-9)16-12-4-2-1-3-7-17(12)13(10)15/h5-6,8,15H,1-4,7H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50289101
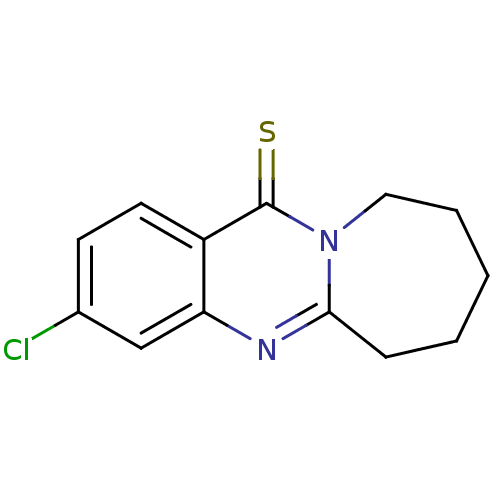
(3-Chloro-12-thioxo-6,7,8,9,10,12-hexahydro-azepino...)Show InChI InChI=1S/C13H13ClN2S/c14-9-5-6-10-11(8-9)15-12-4-2-1-3-7-16(12)13(10)17/h5-6,8H,1-4,7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50289093
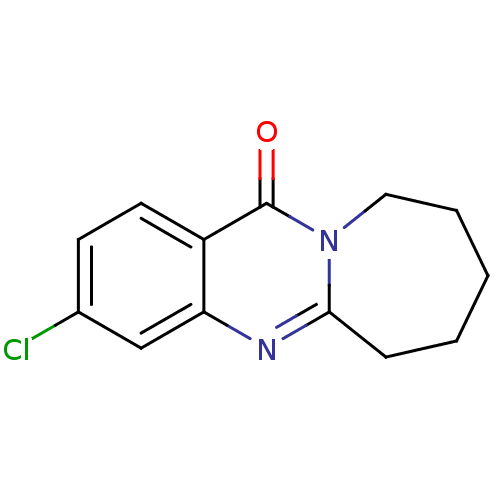
(3-Chloro-12-oxo-6,7,8,9,10,12-hexahydro-azepino[2,...)Show InChI InChI=1S/C13H13ClN2O/c14-9-5-6-10-11(8-9)15-12-4-2-1-3-7-16(12)13(10)17/h5-6,8H,1-4,7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in human RBC |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50289101

(3-Chloro-12-thioxo-6,7,8,9,10,12-hexahydro-azepino...)Show InChI InChI=1S/C13H13ClN2S/c14-9-5-6-10-11(8-9)15-12-4-2-1-3-7-16(12)13(10)17/h5-6,8H,1-4,7H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50289093

(3-Chloro-12-oxo-6,7,8,9,10,12-hexahydro-azepino[2,...)Show InChI InChI=1S/C13H13ClN2O/c14-9-5-6-10-11(8-9)15-12-4-2-1-3-7-16(12)13(10)17/h5-6,8H,1-4,7H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50289097

(6,7,8,9,10,11,12,13-Octahydro-5,12a-diaza-cyclonon...)Show InChI InChI=1S/C15H20N2/c1-2-4-10-15-16-14-9-6-5-8-13(14)12-17(15)11-7-3-1/h5-6,8-9H,1-4,7,10-12H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50289098

(6,8,9,10,11,12-Hexahydro-7H-5,11a-diaza-cycloocta[...)Show InChI InChI=1S/C14H18N2/c1-2-6-10-16-11-12-7-4-5-8-13(12)15-14(16)9-3-1/h4-5,7-8H,1-3,6,9-11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50289095

(6,8,9,11-Tetrahydro-7H-pyrido[2,1-b]quinazoline | ...)Show InChI InChI=1S/C12H14N2/c1-2-6-11-10(5-1)9-14-8-4-3-7-12(14)13-11/h1-2,5-6H,3-4,7-9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase in rat brain |
Bioorg Med Chem Lett 6: 737-742 (1996)
Article DOI: 10.1016/0960-894X(96)00102-3
BindingDB Entry DOI: 10.7270/Q2736RDK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data