

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016848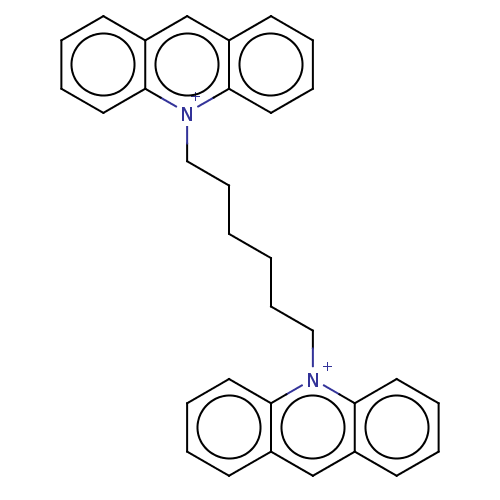 (CHEMBL3276408) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016848 (CHEMBL3276408) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016849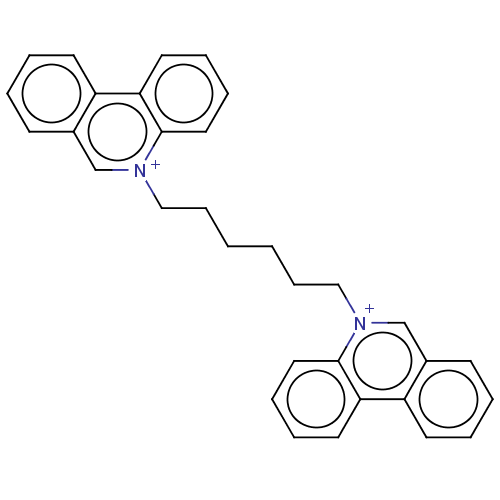 (CHEMBL3276410) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016849 (CHEMBL3276410) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016848 (CHEMBL3276408) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016850 (CHEMBL3276411) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016875 (CHEMBL3276431) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor com... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016871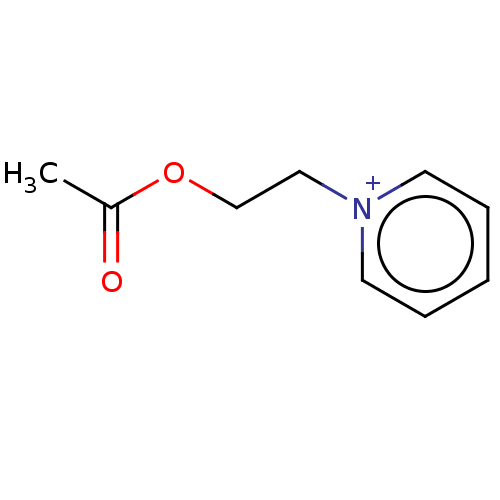 (CHEMBL3276432) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor com... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016871 (CHEMBL3276432) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor com... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016875 (CHEMBL3276431) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor com... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016848 (CHEMBL3276408) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016849 (CHEMBL3276410) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016828 (CHEMBL3276418) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016851 (CHEMBL3276412) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016827 (CHEMBL3276417) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016850 (CHEMBL3276411) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016849 (CHEMBL3276410) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016872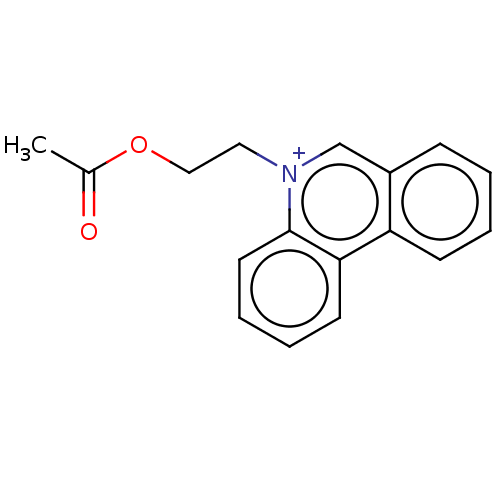 (CHEMBL3276421) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor com... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016870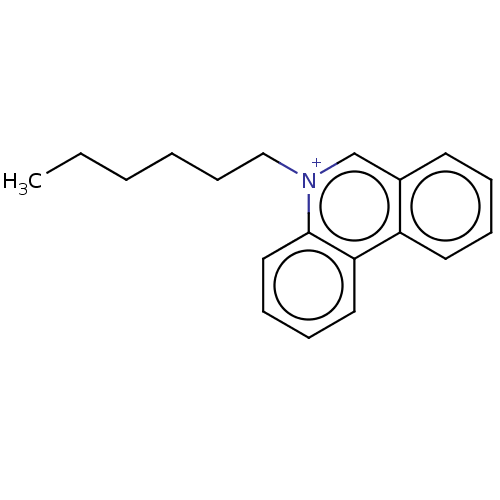 (CHEMBL3276416) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016851 (CHEMBL3276412) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM11023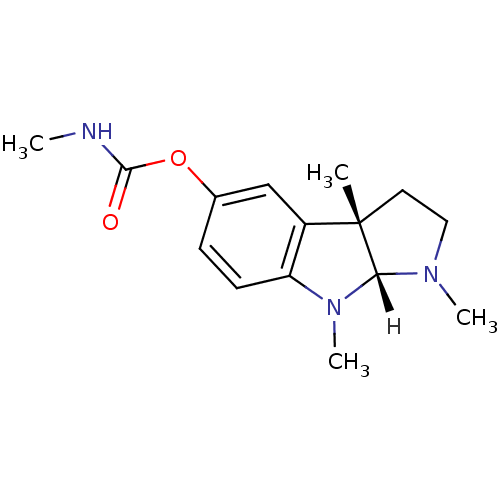 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016851 (CHEMBL3276412) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM120263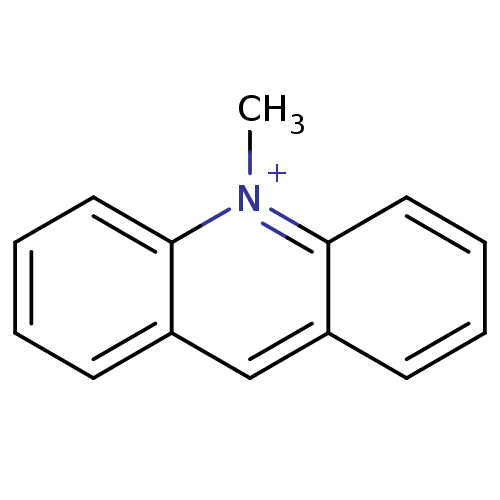 (N-methylacridinium) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016827 (CHEMBL3276417) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016827 (CHEMBL3276417) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016828 (CHEMBL3276418) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016828 (CHEMBL3276418) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016845 (HEXAFLUORENIUM BROMIDE | Hexaflurone Bromide | Hex...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016850 (CHEMBL3276411) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016872 (CHEMBL3276421) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor com... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM120263 (N-methylacridinium) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016870 (CHEMBL3276416) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016851 (CHEMBL3276412) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016828 (CHEMBL3276418) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016873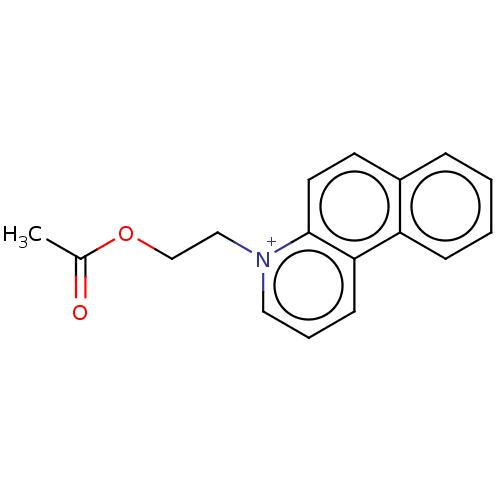 (CHEMBL3276426) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor com... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016838 (CHEMBL3276423) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016845 (HEXAFLUORENIUM BROMIDE | Hexaflurone Bromide | Hex...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016870 (CHEMBL3276416) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016840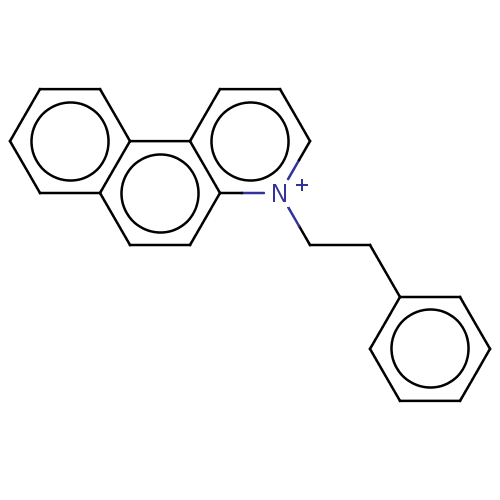 (CHEMBL3276424) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016873 (CHEMBL3276426) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible competitive inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor com... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016827 (CHEMBL3276417) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016852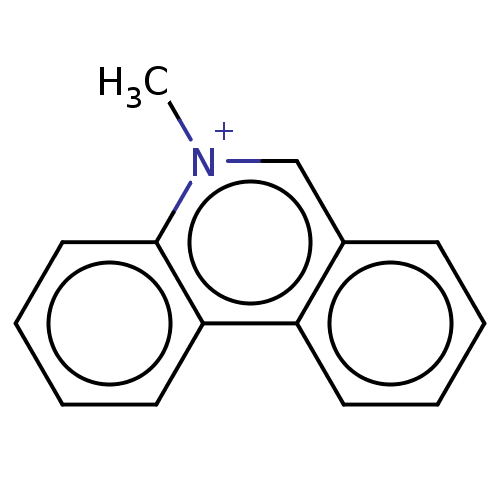 (CHEMBL3276413) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016845 (HEXAFLUORENIUM BROMIDE | Hexaflurone Bromide | Hex...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016856 (CHEMBL3276414) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016845 (HEXAFLUORENIUM BROMIDE | Hexaflurone Bromide | Hex...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016844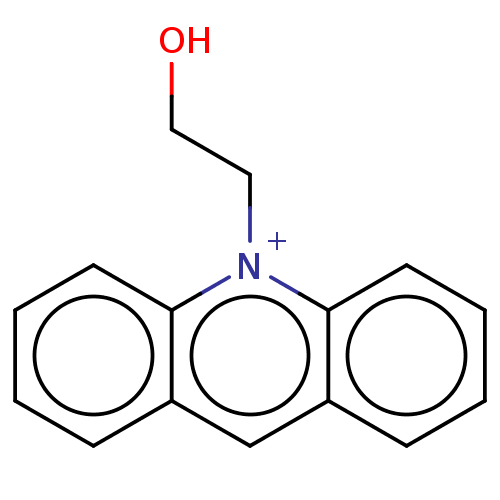 (CHEMBL3276430) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016850 (CHEMBL3276411) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016874 (CHEMBL3276409) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor complex by Line... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016864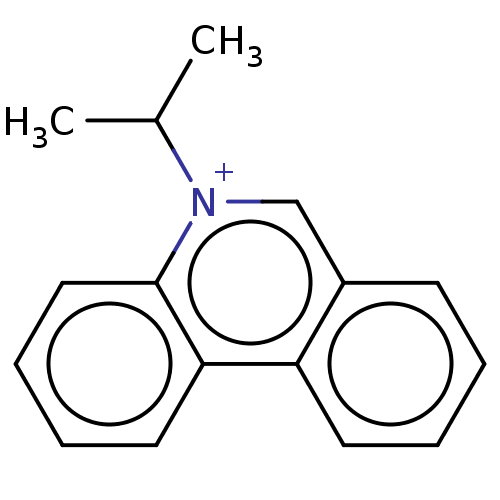 (CHEMBL3276415) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM120263 (N-methylacridinium) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016840 (CHEMBL3276424) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016870 (CHEMBL3276416) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM120263 (N-methylacridinium) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016844 (CHEMBL3276430) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016856 (CHEMBL3276414) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016864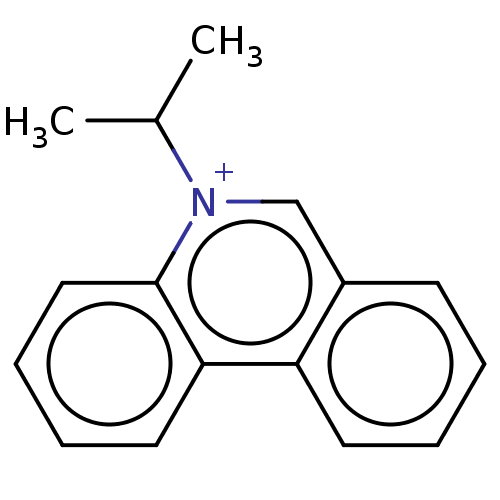 (CHEMBL3276415) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016840 (CHEMBL3276424) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016837 (CHEMBL3276422) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016852 (CHEMBL3276413) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016838 (CHEMBL3276423) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016852 (CHEMBL3276413) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016844 (CHEMBL3276430) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016856 (CHEMBL3276414) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016874 (CHEMBL3276409) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016837 (CHEMBL3276422) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016838 (CHEMBL3276423) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016844 (CHEMBL3276430) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016836 (CHEMBL3276420) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016856 (CHEMBL3276414) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016842 (CHEMBL3276427) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016840 (CHEMBL3276424) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016864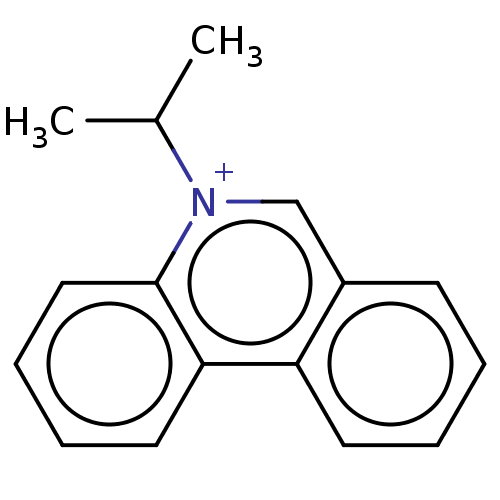 (CHEMBL3276415) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016837 (CHEMBL3276422) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016852 (CHEMBL3276413) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | PubMed | 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016831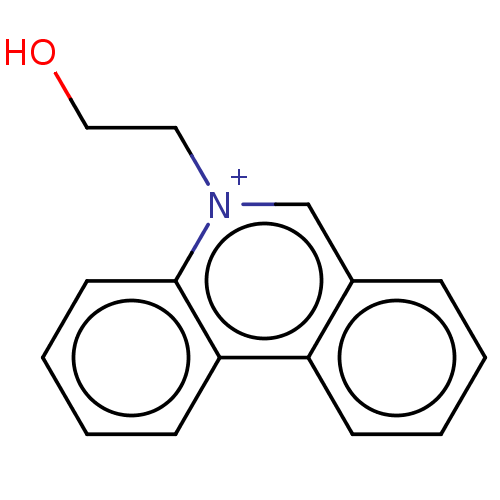 (CHEMBL3276419) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016842 (CHEMBL3276427) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016837 (CHEMBL3276422) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016831 (CHEMBL3276419) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016838 (CHEMBL3276423) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016874 (CHEMBL3276409) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor complex by Line... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016836 (CHEMBL3276420) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016831 (CHEMBL3276419) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016836 (CHEMBL3276420) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016841 (CHEMBL3276425) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016841 (CHEMBL3276425) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016836 (CHEMBL3276420) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016864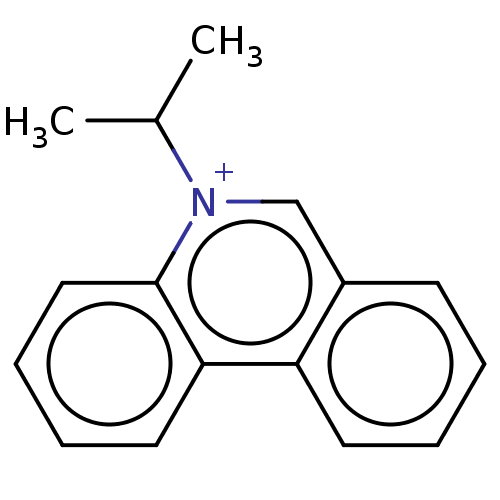 (CHEMBL3276415) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016831 (CHEMBL3276419) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016843 (CHEMBL3276428) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016871 (CHEMBL3276432) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016871 (CHEMBL3276432) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor complex by Line... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50119784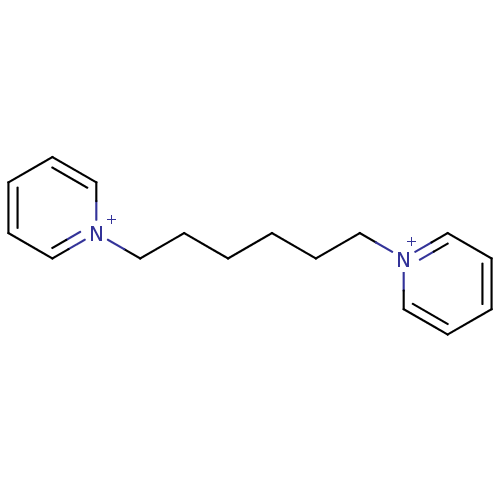 (1-[6-(1lambda~5~-pyridin-1-yl)hexyl]-1lambda~5~-py...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor complex by Line... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016874 (CHEMBL3276409) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016842 (CHEMBL3276427) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016843 (CHEMBL3276428) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016841 (CHEMBL3276425) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016841 (CHEMBL3276425) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016842 (CHEMBL3276427) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016871 (CHEMBL3276432) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 3.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016871 (CHEMBL3276432) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 3.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor complex by Line... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50016843 (CHEMBL3276428) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50016843 (CHEMBL3276428) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50119784 (1-[6-(1lambda~5~-pyridin-1-yl)hexyl]-1lambda~5~-py...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50119784 (1-[6-(1lambda~5~-pyridin-1-yl)hexyl]-1lambda~5~-py...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as concentration required for 25% inhibi... | J Med Chem 20: 1617-23 (1978) BindingDB Entry DOI: 10.7270/Q29025BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||