

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027613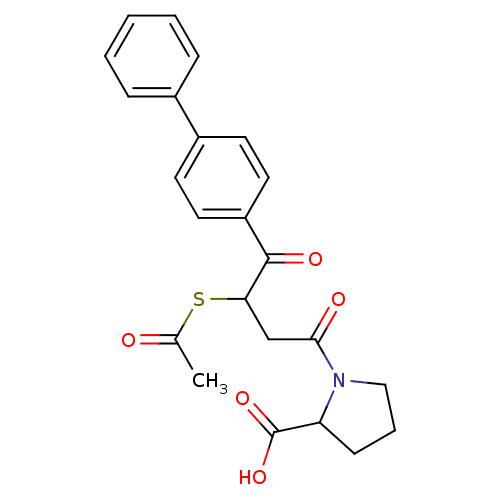 (1-(3-Acetylsulfanyl-4-biphenyl-4-yl-4-oxo-butyryl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027608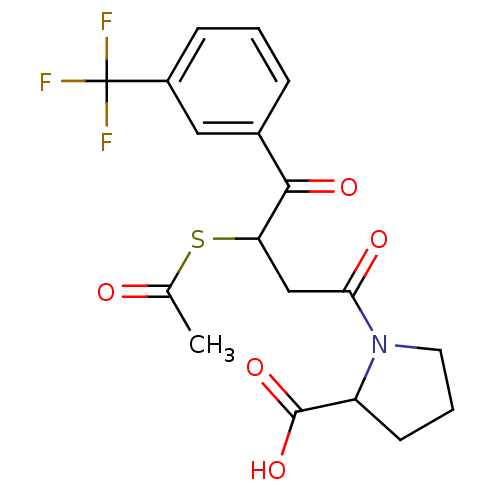 (1-[3-Acetylsulfanyl-4-oxo-4-(3-trifluoromethyl-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027628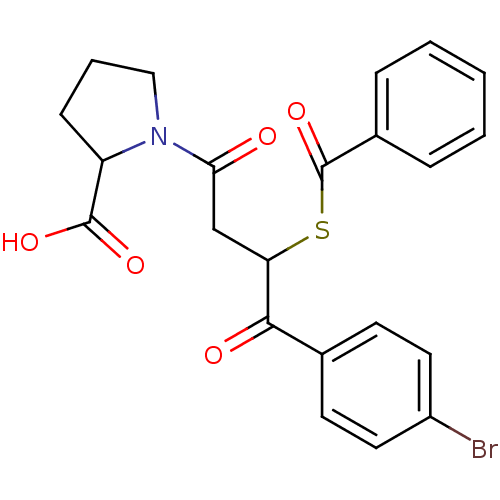 (1-[3-Benzoylsulfanyl-4-(4-bromo-phenyl)-4-oxo-buty...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027629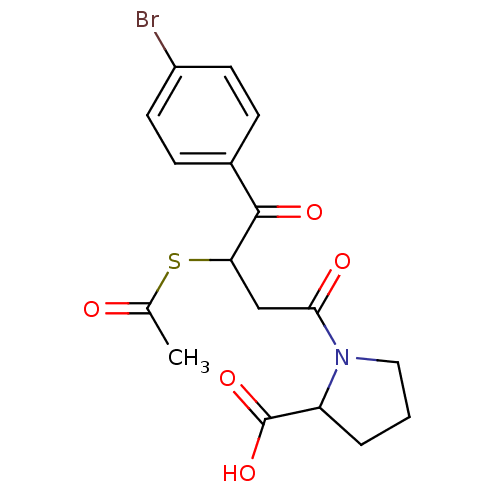 (1-[3-Acetylsulfanyl-4-(4-bromo-phenyl)-4-oxo-butyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027610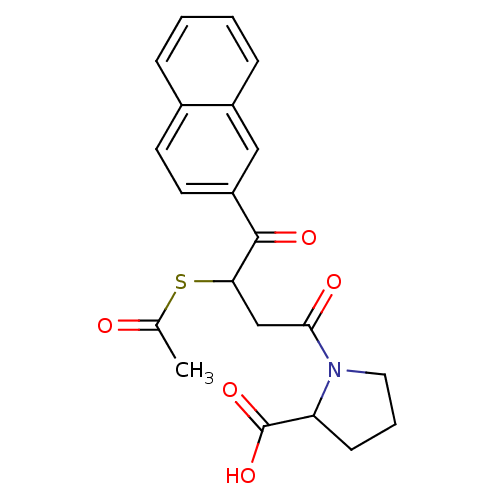 (1-(3-Acetylsulfanyl-4-naphthalen-2-yl-4-oxo-butyry...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027619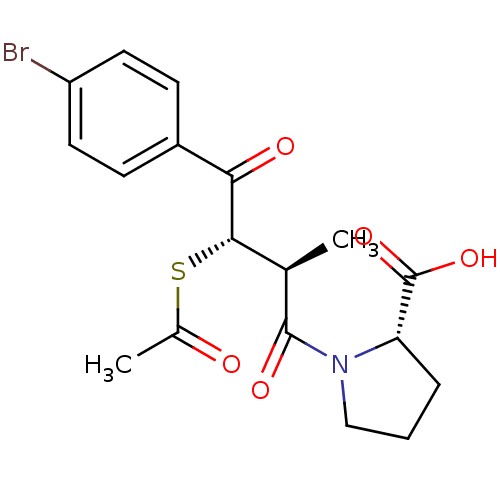 (1-[3-Acetylsulfanyl-4-(4-bromo-phenyl)-2-methyl-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027605 (1-[3-Benzoylsulfanyl-4-(3-fluoro-phenyl)-4-oxo-but...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027612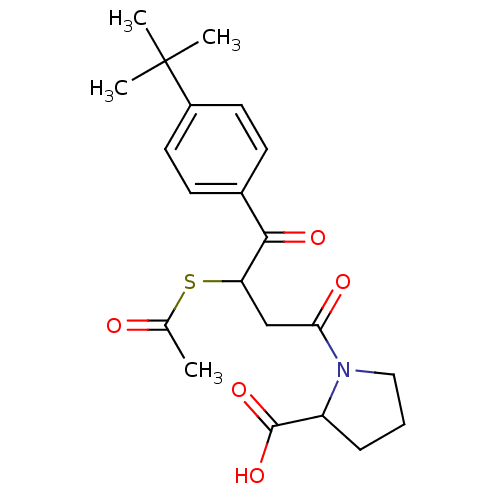 (1-[3-Acetylsulfanyl-4-(4-tert-butyl-phenyl)-4-oxo-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 183 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027606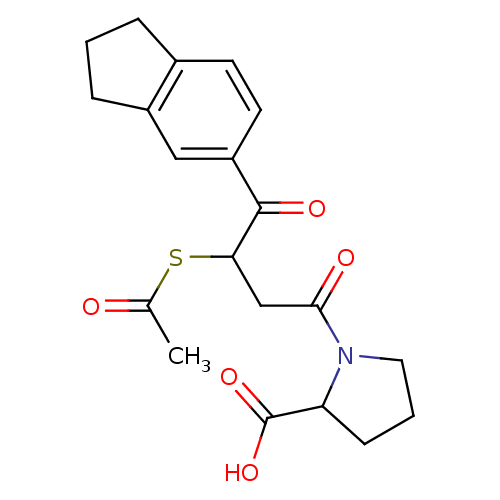 (1-(3-Acetylsulfanyl-4-indan-5-yl-4-oxo-butyryl)-py...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027620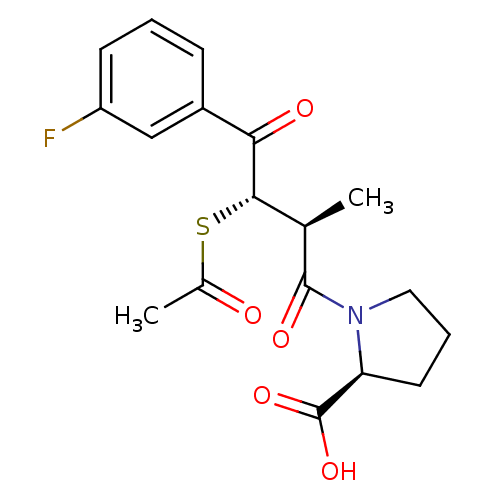 (1-[3-Acetylsulfanyl-4-(3-fluoro-phenyl)-2-methyl-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027607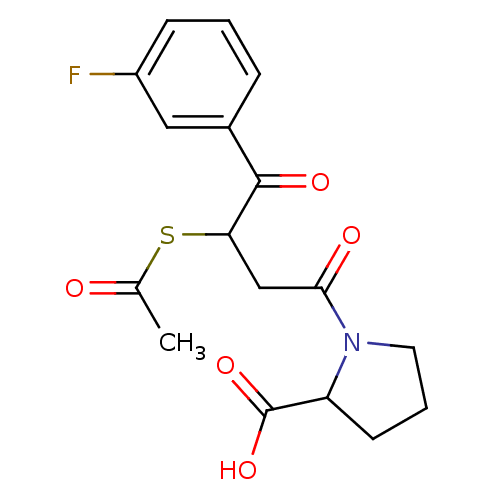 (1-[3-Acetylsulfanyl-4-(3-fluoro-phenyl)-4-oxo-buty...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 317 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027603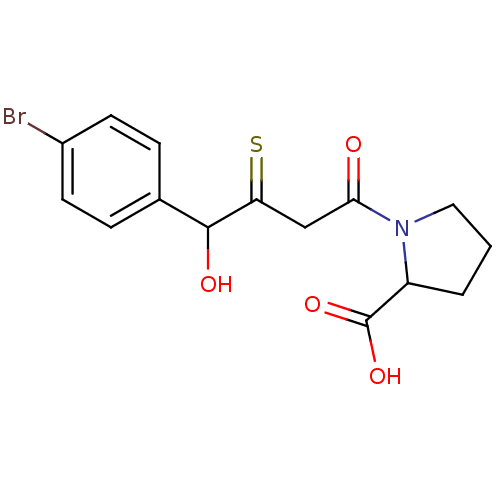 (1-[4-(4-Bromo-phenyl)-3-mercapto-4-oxo-butyryl]-py...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 352 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027618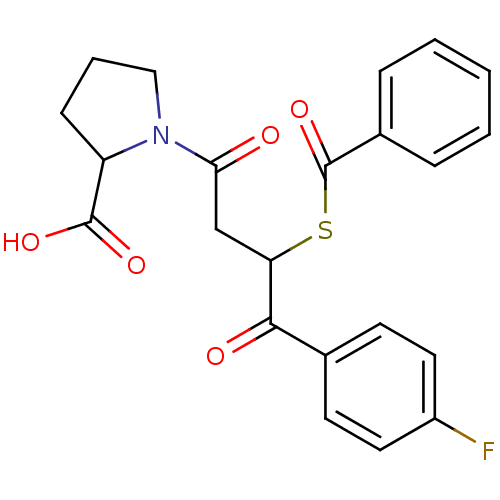 (1-[3-Benzoylsulfanyl-4-(4-fluoro-phenyl)-4-oxo-but...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 353 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027626 (1-{3-Acetylsulfanyl-4-[4-(4-chloro-phenoxy)-phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 358 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027609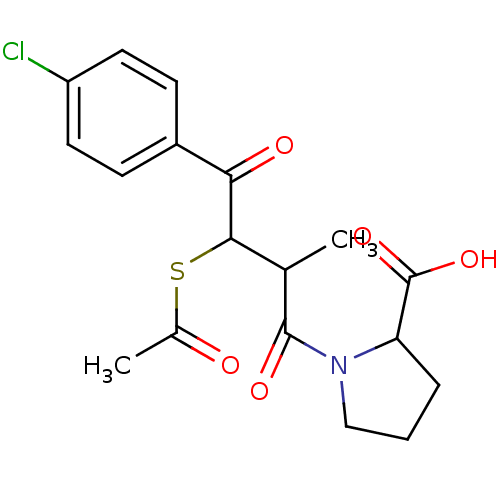 (1-[3-Acetylsulfanyl-4-(4-chloro-phenyl)-2-methyl-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 365 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027614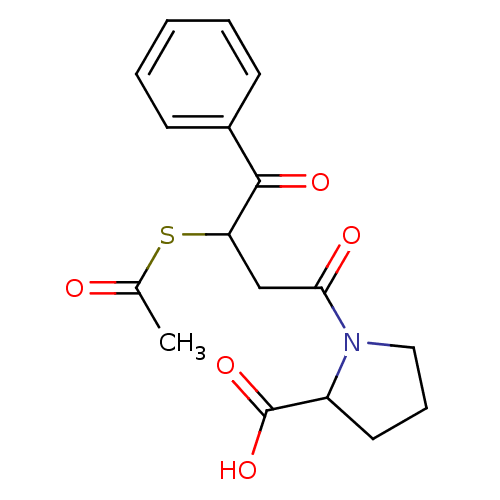 (1-(3-Acetylsulfanyl-4-oxo-4-phenyl-butyryl)-pyrrol...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 455 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027624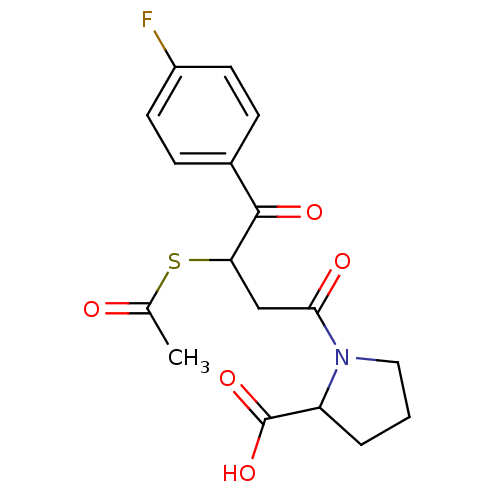 (1-[3-Acetylsulfanyl-4-(4-fluoro-phenyl)-4-oxo-buty...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 473 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027611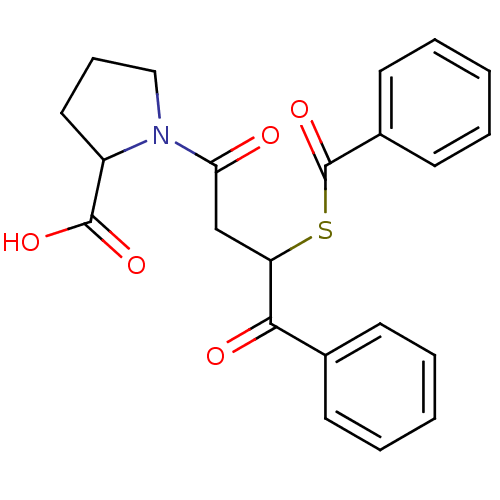 (1-(3-Benzoylsulfanyl-4-oxo-4-phenyl-butyryl)-pyrro...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027623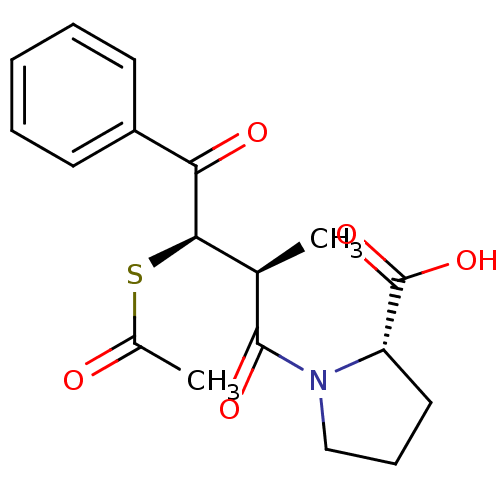 (1-(3-Acetylsulfanyl-2-methyl-4-oxo-4-phenyl-butyry...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 549 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027617 (1-[3-Acetylsulfanyl-4-(3-fluoro-4-methoxy-phenyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027627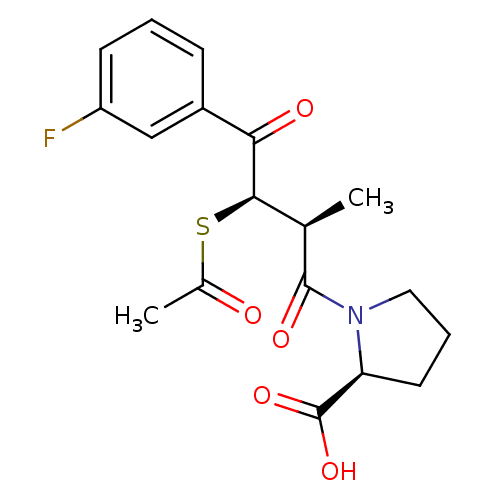 (1-[3-Acetylsulfanyl-4-(3-fluoro-phenyl)-2-methyl-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 617 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027615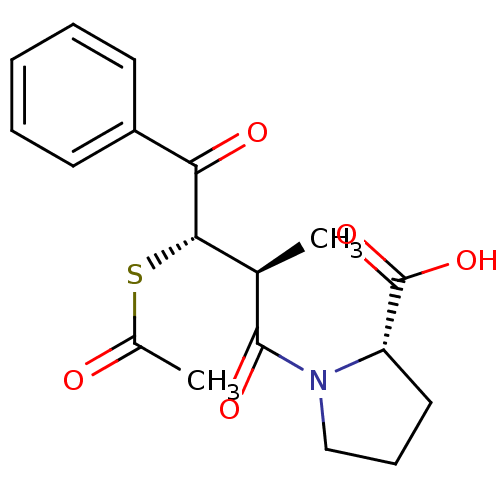 (1-(3-Acetylsulfanyl-2-methyl-4-oxo-4-phenyl-butyry...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027616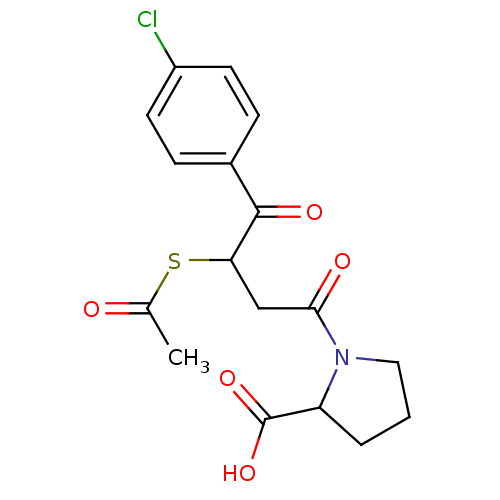 (1-[3-Acetylsulfanyl-4-(4-chloro-phenyl)-4-oxo-buty...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 825 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027625 (1-(3-Acetylsulfanyl-2-methyl-4-naphthalen-1-yl-4-o...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027604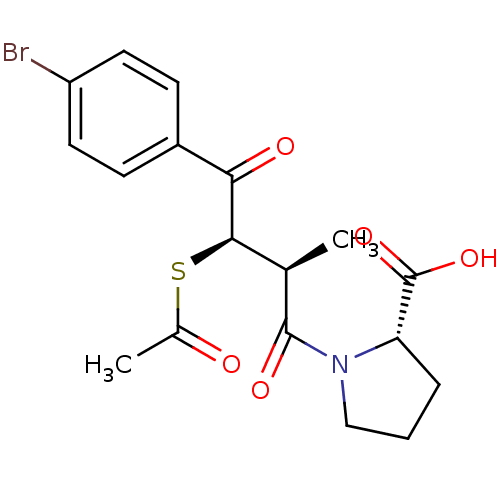 (1-[3-Acetylsulfanyl-4-(4-bromo-phenyl)-2-methyl-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 985 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027622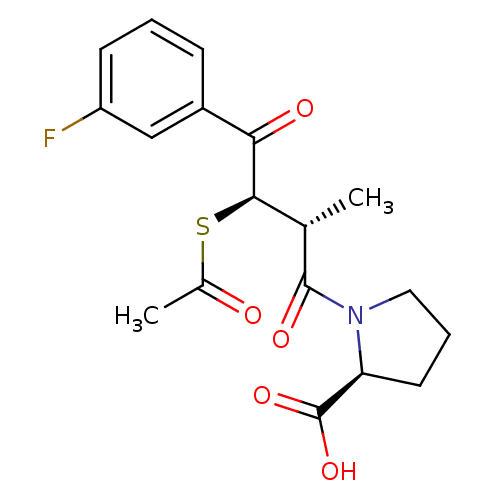 (1-[3-Acetylsulfanyl-4-(3-fluoro-phenyl)-2-methyl-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50027621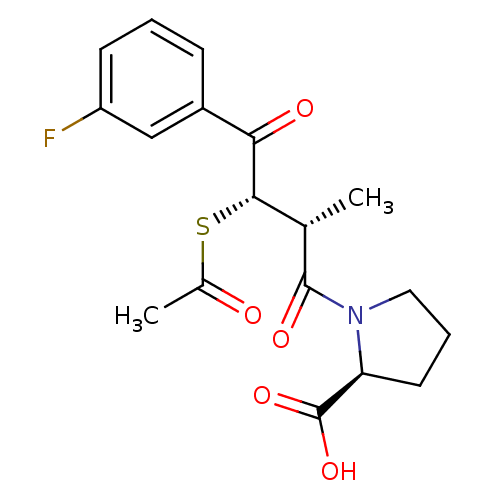 (1-[3-Acetylsulfanyl-4-(3-fluoro-phenyl)-2-methyl-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antihypertensive activity determined by inhibition of angiotensin I converting enzyme | J Med Chem 26: 381-93 (1983) BindingDB Entry DOI: 10.7270/Q2XS5VZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||