

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (MOUSE) | BDBM50027791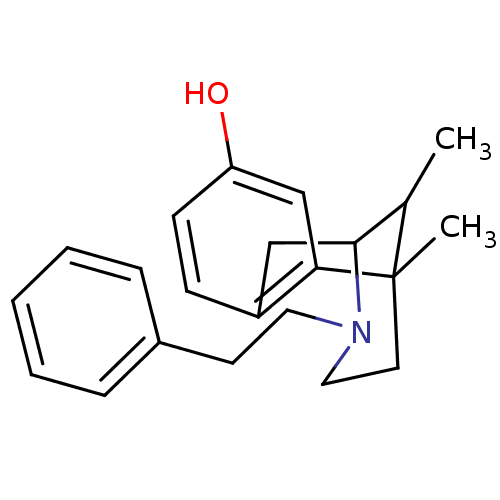 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to induce 50% of maximal effect in mouse vas deferens expressing Opioid receptor delta 1 | J Med Chem 26: 1643-5 (1983) BindingDB Entry DOI: 10.7270/Q2N015J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50000091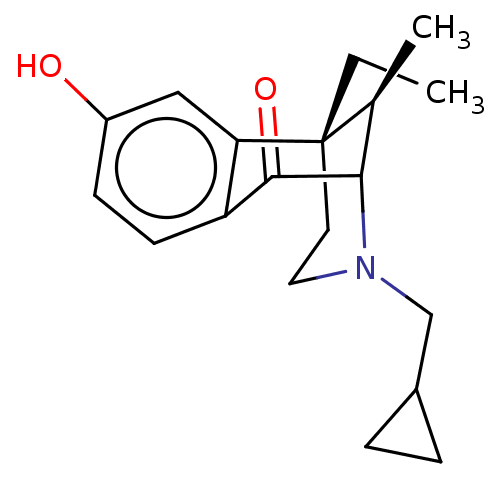 ((6S,11R)-3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace 50% of (+/-) ethylketocyclazocine (2.8) in rat brain homogenate | J Med Chem 26: 1643-5 (1983) BindingDB Entry DOI: 10.7270/Q2N015J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50027790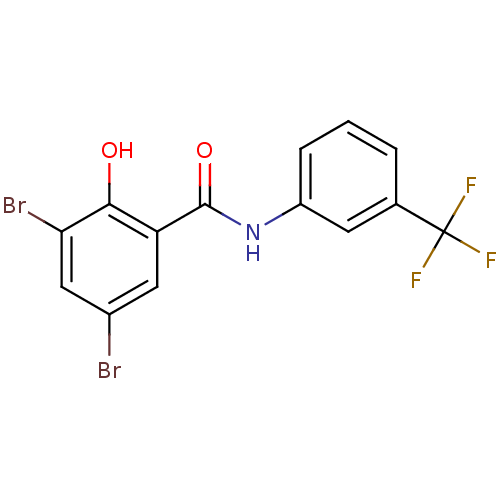 (3,5-Dibromo-2-hydroxy-N-(3-trifluoromethyl-phenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to induce 50% of maximal effect in mouse vas deferens expressing Opioid receptor delta 1 | J Med Chem 26: 1643-5 (1983) BindingDB Entry DOI: 10.7270/Q2N015J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50001465 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to induce 50% of maximal effect in mouse vas deferens expressing Opioid receptor delta 1 | J Med Chem 26: 1643-5 (1983) BindingDB Entry DOI: 10.7270/Q2N015J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50027791 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to induce 50% of maximal effect in guinea pig ileum expressing Opioid receptor mu 1 | J Med Chem 26: 1643-5 (1983) BindingDB Entry DOI: 10.7270/Q2N015J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50027790 (3,5-Dibromo-2-hydroxy-N-(3-trifluoromethyl-phenyl)...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to induce 50% of maximal effect in guinea pig ileum expressing Opioid receptor mu 1 | J Med Chem 26: 1643-5 (1983) BindingDB Entry DOI: 10.7270/Q2N015J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000091 ((6S,11R)-3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to induce 50% of maximal effect in rabbit vas deferens expressing Opioid receptor kappa 1 | J Med Chem 26: 1643-5 (1983) BindingDB Entry DOI: 10.7270/Q2N015J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to induce 50% of maximal effect in guinea pig ileum expressing Opioid receptor mu 1 | J Med Chem 26: 1643-5 (1983) BindingDB Entry DOI: 10.7270/Q2N015J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027791 (6,11-Dimethyl-3-phenethyl-1,2,3,4,5,6-hexahydro-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to induce 50% of maximal effect in rabbit vas deferens expressing Opioid receptor kappa 1 | J Med Chem 26: 1643-5 (1983) BindingDB Entry DOI: 10.7270/Q2N015J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027790 (3,5-Dibromo-2-hydroxy-N-(3-trifluoromethyl-phenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to induce 50% of maximal effect in rabbit vas deferens expressing Opioid receptor kappa 1 | J Med Chem 26: 1643-5 (1983) BindingDB Entry DOI: 10.7270/Q2N015J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||