

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pepsin A (Porcine) | BDBM50028313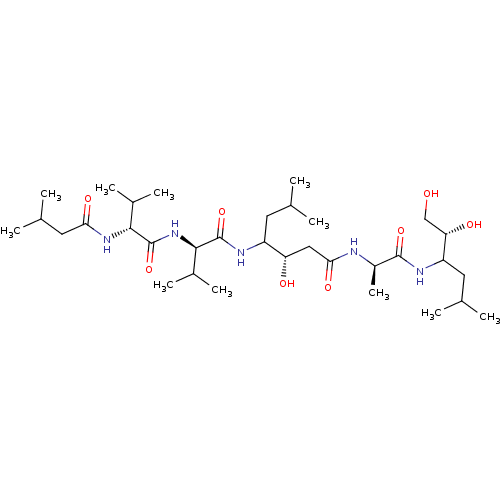 (3-Hydroxy-6-methyl-4-{3-methyl-2-[3-methyl-2-(3-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Pepsin inhibition was measured using the synthetic heptapeptide substrate Phe-Gly-His-Phe-(N02)-Phe-Ala- Phe-OMe | J Med Chem 23: 27-33 (1980) BindingDB Entry DOI: 10.7270/Q24T6HCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50028314 (CHEMBL325506 | N-(3-{1-Hydroxy-2-[1-(3-methyl-buty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Pepsin inhibition was measured using the synthetic heptapeptide substrate Phe-Gly-His-Phe-(N02)-Phe-Ala- Phe-OMe | J Med Chem 23: 27-33 (1980) BindingDB Entry DOI: 10.7270/Q24T6HCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50028312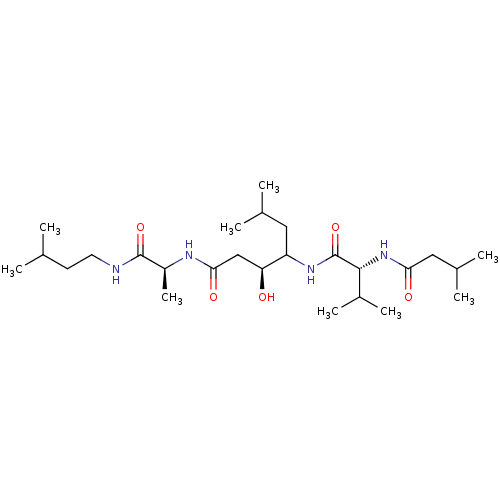 (3-Hydroxy-6-methyl-4-[3-methyl-2-(3-methyl-butyryl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Pepsin inhibition was measured using the synthetic heptapeptide substrate Phe-Gly-His-Phe-(N02)-Phe-Ala- Phe-OMe | J Med Chem 23: 27-33 (1980) BindingDB Entry DOI: 10.7270/Q24T6HCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50028311 (3-Hydroxy-6-methyl-4-(3-methyl-butyrylamino)-hepta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Pepsin inhibition was measured using the synthetic heptapeptide substrate Phe-Gly-His-Phe-(N02)-Phe-Ala- Phe-OMe | J Med Chem 23: 27-33 (1980) BindingDB Entry DOI: 10.7270/Q24T6HCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A-5 (Homo sapiens (Human)) | BDBM50028318 (3-Hydroxy-6-methyl-4-[3-methyl-2-(3-methyl-butyryl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Pepsin inhibition using synthetic heptapeptide substrate Phe-Gly-His-Phe-(N02)-Phe-Ala- Phe-OMe | J Med Chem 23: 27-33 (1980) BindingDB Entry DOI: 10.7270/Q24T6HCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A-5 (Homo sapiens (Human)) | BDBM50028315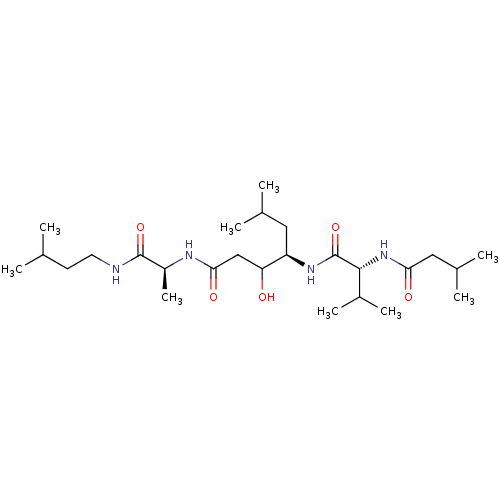 (3-Hydroxy-6-methyl-4-[3-methyl-2-(3-methyl-butyryl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Pepsin inhibition using synthetic heptapeptide substrate Phe-Gly-His-Phe-(N02)-Phe-Ala- Phe-OMe | J Med Chem 23: 27-33 (1980) BindingDB Entry DOI: 10.7270/Q24T6HCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pepsin A (Porcine) | BDBM50028316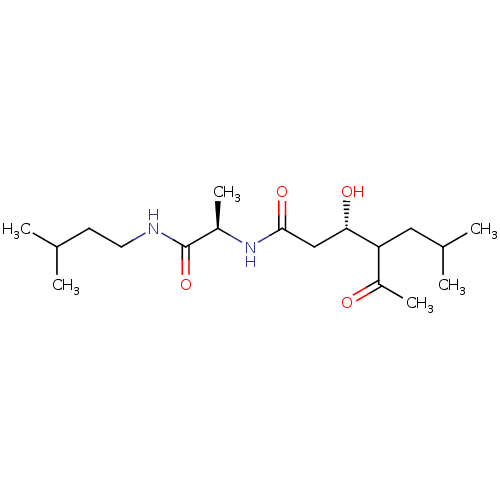 (4-Acetyl-3-hydroxy-6-methyl-heptanoic acid [1-(3-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Pepsin inhibition was measured using the synthetic heptapeptide substrate Phe-Gly-His-Phe-(N02)-Phe-Ala- Phe-OMe | J Med Chem 23: 27-33 (1980) BindingDB Entry DOI: 10.7270/Q24T6HCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50028313 (3-Hydroxy-6-methyl-4-{3-methyl-2-[3-methyl-2-(3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of renin was measured using hog kidney reninand the radiolabeled synthetic substrate, H-Ile-His-Pro-Phe-His-Leu-[14C]Leu-Val-Tyr-Ser-OH | J Med Chem 23: 27-33 (1980) BindingDB Entry DOI: 10.7270/Q24T6HCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50028314 (CHEMBL325506 | N-(3-{1-Hydroxy-2-[1-(3-methyl-buty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of renin was measured using hog kidney and the radiolabeled synthetic substrate, H-Ile-His-Pro-Phe-His-Leu-[14C]Leu-Val-Tyr-Ser-OH | J Med Chem 23: 27-33 (1980) BindingDB Entry DOI: 10.7270/Q24T6HCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50028312 (3-Hydroxy-6-methyl-4-[3-methyl-2-(3-methyl-butyryl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of renin was measured using hog kidney and the radiolabeled synthetic substrate, H-Ile-His-Pro-Phe-His-Leu-[14C]Leu-Val-Tyr-Ser-OH | J Med Chem 23: 27-33 (1980) BindingDB Entry DOI: 10.7270/Q24T6HCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50028316 (4-Acetyl-3-hydroxy-6-methyl-heptanoic acid [1-(3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of renin was measured using hog kidney reninand the radiolabeled synthetic substrate, H-Ile-His-Pro-Phe-His-Leu-[14C]Leu-Val-Tyr-Ser-OH | J Med Chem 23: 27-33 (1980) BindingDB Entry DOI: 10.7270/Q24T6HCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50028319 (3-Hydroxy-6-methyl-4-{3-methyl-2-[3-methyl-2-(3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of renin was measured using hog kidney and the radiolabeled synthetic substrate, H-Ile-His-Pro-Phe-His-Leu-[14C]Leu-Val-Tyr-Ser-OH | J Med Chem 23: 27-33 (1980) BindingDB Entry DOI: 10.7270/Q24T6HCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50028317 (3-Hydroxy-6-methyl-4-[3-methyl-2-(3-methyl-butyryl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of renin was measured using hog kidney reninand the radiolabeled synthetic substrate, H-Ile-His-Pro-Phe-His-Leu-[14C]Leu-Val-Tyr-Ser-OH | J Med Chem 23: 27-33 (1980) BindingDB Entry DOI: 10.7270/Q24T6HCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50028311 (3-Hydroxy-6-methyl-4-(3-methyl-butyrylamino)-hepta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of renin was measured using hog kidney reninand the radiolabeled synthetic substrate, H-Ile-His-Pro-Phe-His-Leu-[14C]Leu-Val-Tyr-Ser-OH | J Med Chem 23: 27-33 (1980) BindingDB Entry DOI: 10.7270/Q24T6HCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||