

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037393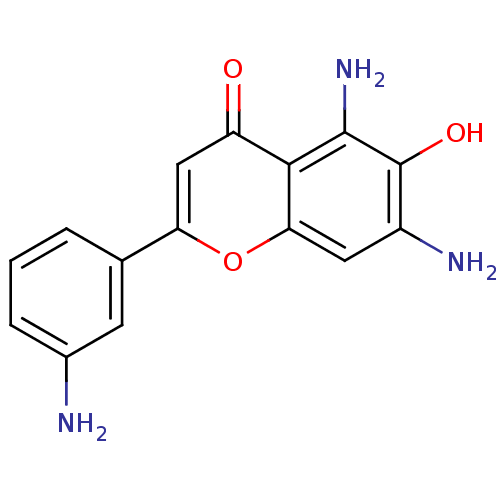 (5,7-Diamino-2-(3-amino-phenyl)-6-hydroxy-chromen-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037413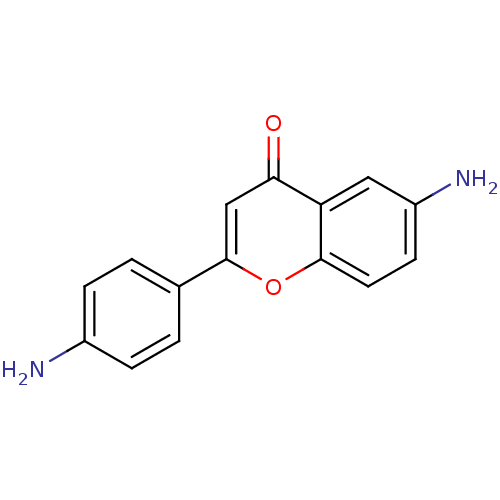 (6-Amino-2-(4-amino-phenyl)-chromen-4-one | CHEMBL1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037408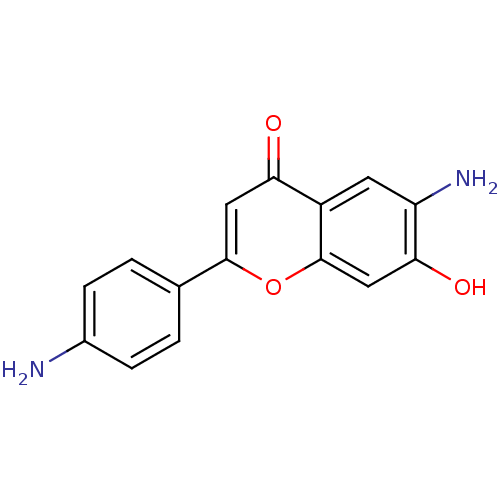 (6-Amino-2-(4-amino-phenyl)-7-hydroxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037395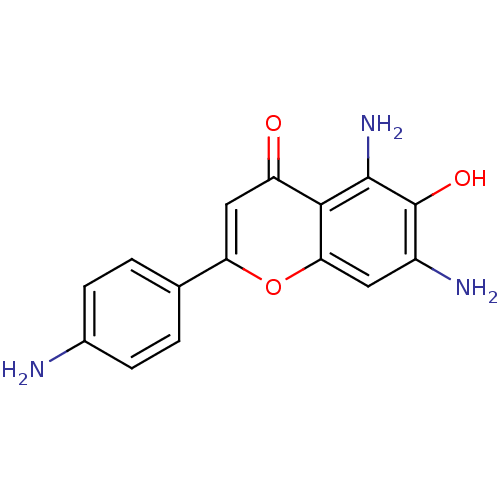 (5,7-Diamino-2-(4-amino-phenyl)-6-hydroxy-chromen-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037413 (6-Amino-2-(4-amino-phenyl)-chromen-4-one | CHEMBL1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037393 (5,7-Diamino-2-(3-amino-phenyl)-6-hydroxy-chromen-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037393 (5,7-Diamino-2-(3-amino-phenyl)-6-hydroxy-chromen-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037410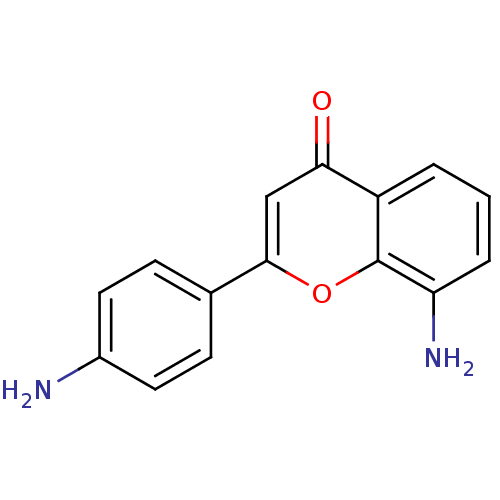 (8-Amino-2-(4-amino-phenyl)-chromen-4-one | CHEMBL3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037396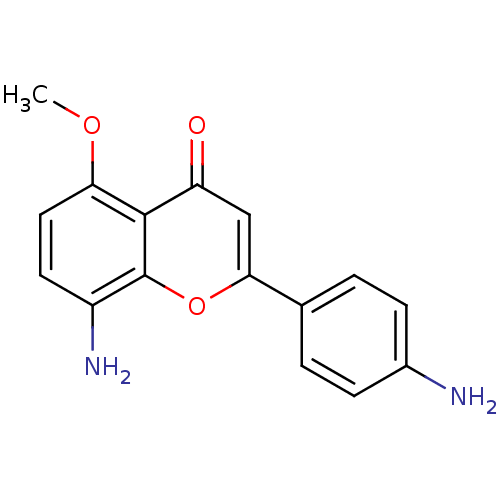 (8-Amino-2-(4-amino-phenyl)-5-methoxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037408 (6-Amino-2-(4-amino-phenyl)-7-hydroxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037405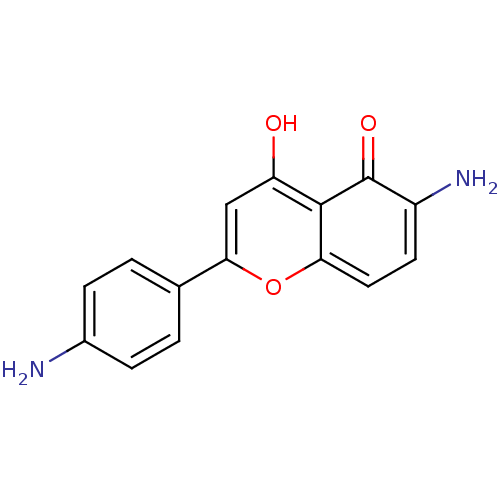 (6-Amino-2-(4-amino-phenyl)-5-hydroxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037392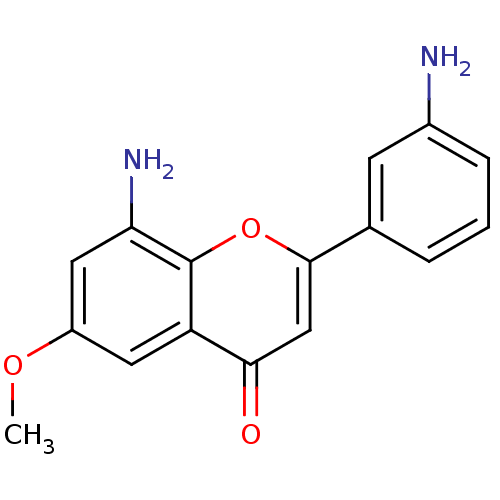 (8-Amino-2-(3-amino-phenyl)-6-methoxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037402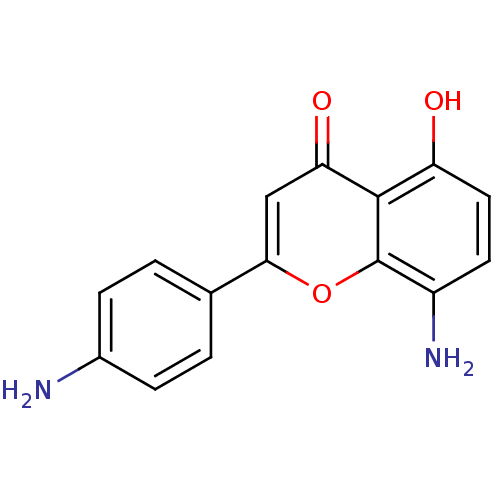 (8-Amino-2-(4-amino-phenyl)-5-hydroxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037395 (5,7-Diamino-2-(4-amino-phenyl)-6-hydroxy-chromen-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037399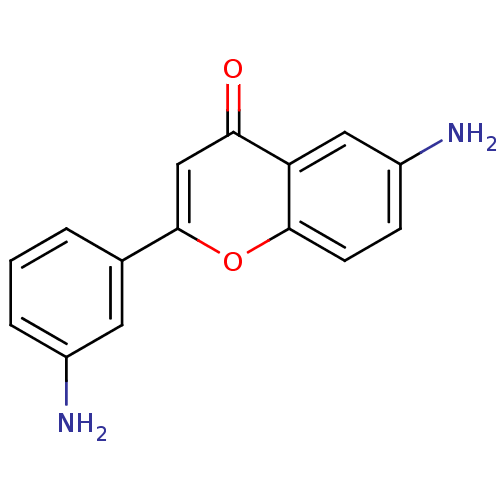 (6-Amino-2-(3-amino-phenyl)-chromen-4-one | CHEMBL1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037407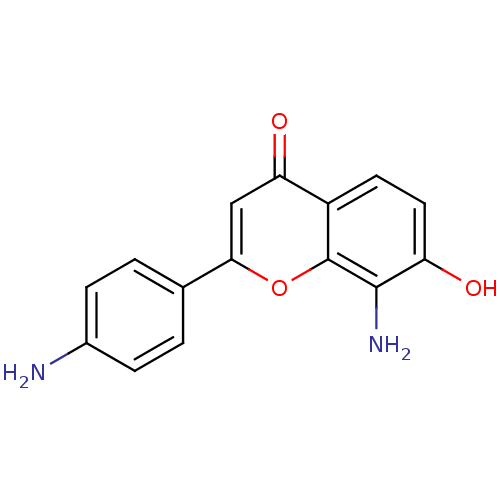 (8-Amino-2-(4-amino-phenyl)-7-hydroxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037401 (8-Amino-2-(4-amino-phenyl)-6-methoxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037397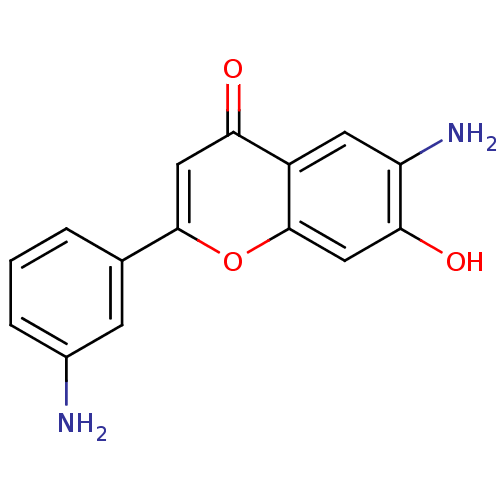 (6-Amino-2-(3-amino-phenyl)-7-hydroxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037406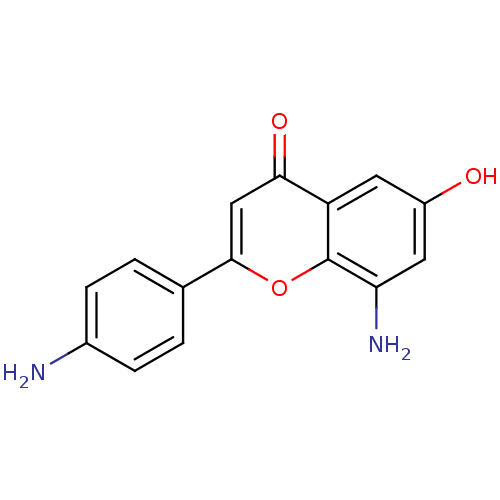 (8-Amino-2-(4-amino-phenyl)-6-hydroxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037409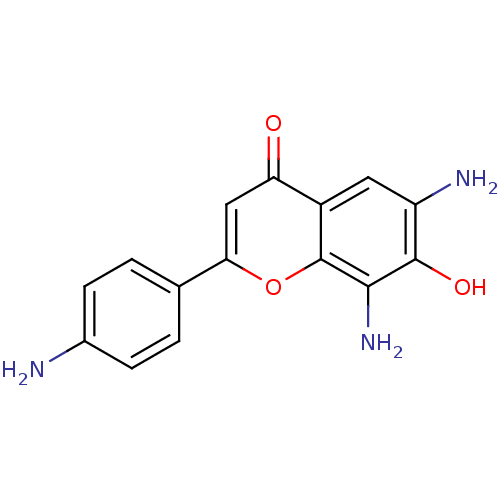 (6,8-Diamino-2-(4-amino-phenyl)-7-hydroxy-chromen-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50037394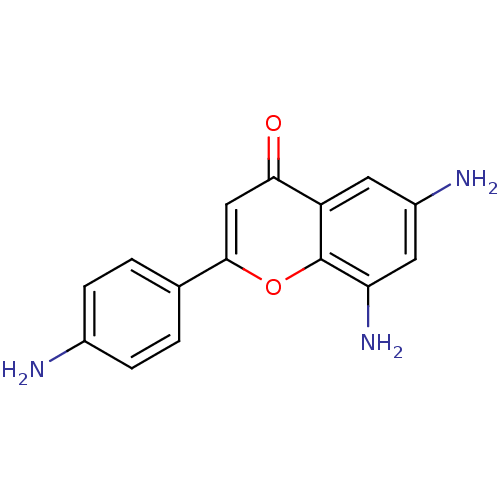 (6,8-Diamino-2-(4-amino-phenyl)-chromen-4-one | CHE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro protein-tyrosine kinase activity of p60v-src enzyme in presence of 5 uM ATP. | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037392 (8-Amino-2-(3-amino-phenyl)-6-methoxy-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037409 (6,8-Diamino-2-(4-amino-phenyl)-7-hydroxy-chromen-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037410 (8-Amino-2-(4-amino-phenyl)-chromen-4-one | CHEMBL3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037394 (6,8-Diamino-2-(4-amino-phenyl)-chromen-4-one | CHE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037392 (8-Amino-2-(3-amino-phenyl)-6-methoxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037397 (6-Amino-2-(3-amino-phenyl)-7-hydroxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037396 (8-Amino-2-(4-amino-phenyl)-5-methoxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037405 (6-Amino-2-(4-amino-phenyl)-5-hydroxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037401 (8-Amino-2-(4-amino-phenyl)-6-methoxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037395 (5,7-Diamino-2-(4-amino-phenyl)-6-hydroxy-chromen-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037402 (8-Amino-2-(4-amino-phenyl)-5-hydroxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037407 (8-Amino-2-(4-amino-phenyl)-7-hydroxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037406 (8-Amino-2-(4-amino-phenyl)-6-hydroxy-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50037399 (6-Amino-2-(3-amino-phenyl)-chromen-4-one | CHEMBL1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of in vitro activity of EGFr enzyme from A431 cells in presence of 5 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037413 (6-Amino-2-(4-amino-phenyl)-chromen-4-one | CHEMBL1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037394 (6,8-Diamino-2-(4-amino-phenyl)-chromen-4-one | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037406 (8-Amino-2-(4-amino-phenyl)-6-hydroxy-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037410 (8-Amino-2-(4-amino-phenyl)-chromen-4-one | CHEMBL3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037408 (6-Amino-2-(4-amino-phenyl)-7-hydroxy-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037399 (6-Amino-2-(3-amino-phenyl)-chromen-4-one | CHEMBL1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037405 (6-Amino-2-(4-amino-phenyl)-5-hydroxy-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.23E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037402 (8-Amino-2-(4-amino-phenyl)-5-hydroxy-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037407 (8-Amino-2-(4-amino-phenyl)-7-hydroxy-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037401 (8-Amino-2-(4-amino-phenyl)-6-methoxy-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037397 (6-Amino-2-(3-amino-phenyl)-7-hydroxy-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037409 (6,8-Diamino-2-(4-amino-phenyl)-7-hydroxy-chromen-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037411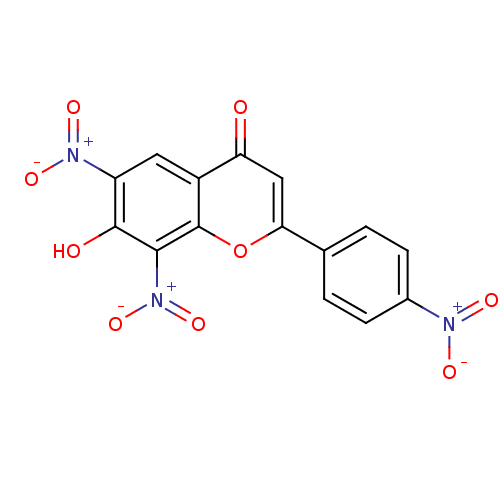 (7-Hydroxy-6,8-dinitro-2-(4-nitro-phenyl)-chromen-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.57E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037396 (8-Amino-2-(4-amino-phenyl)-5-methoxy-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.62E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037417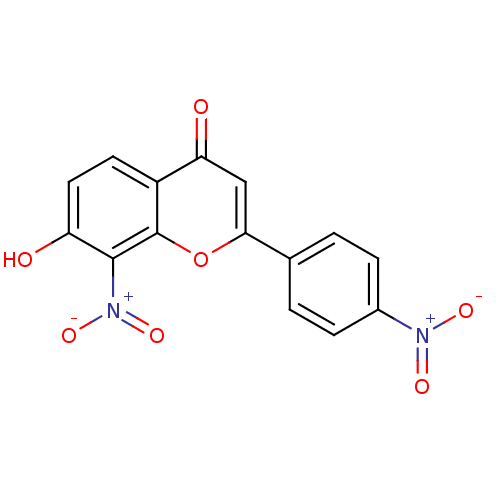 (7-Hydroxy-8-nitro-2-(4-nitro-phenyl)-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.88E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037418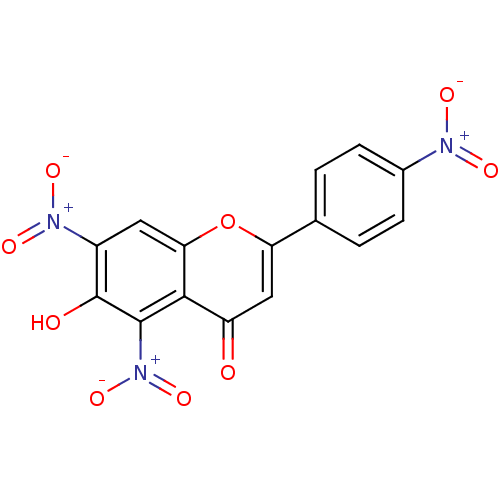 (6-Hydroxy-5,7-dinitro-2-(4-nitro-phenyl)-chromen-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037398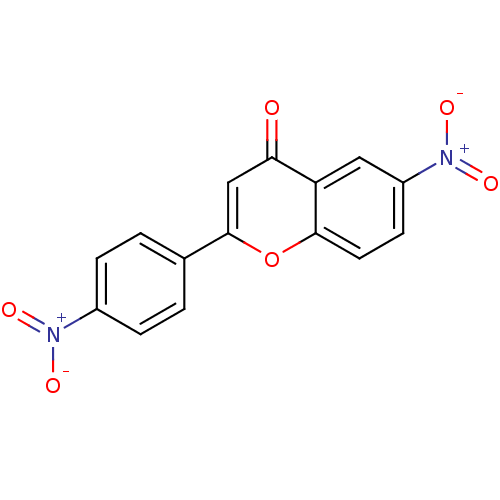 (6-Nitro-2-(4-nitro-phenyl)-chromen-4-one | CHEMBL6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037414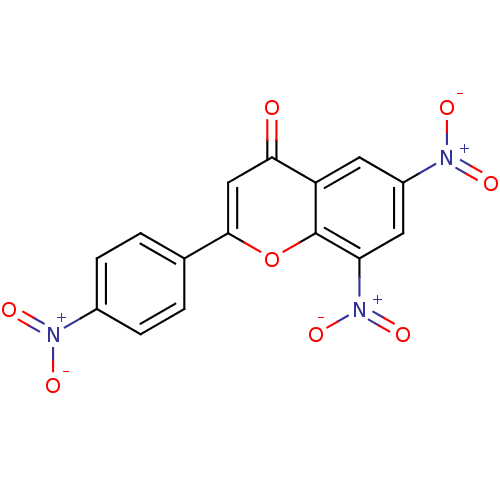 (6,8-Dinitro-2-(4-nitro-phenyl)-chromen-4-one | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037412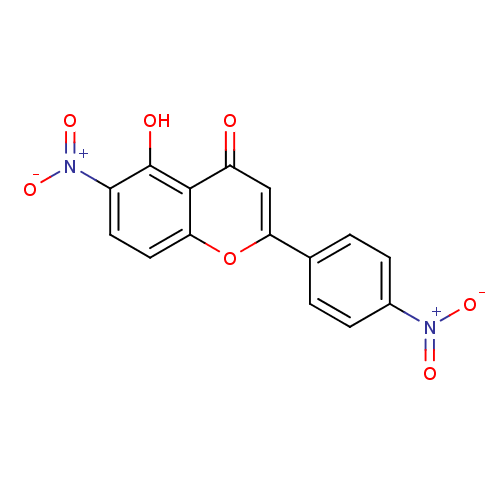 (5-Hydroxy-6-nitro-2-(4-nitro-phenyl)-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037419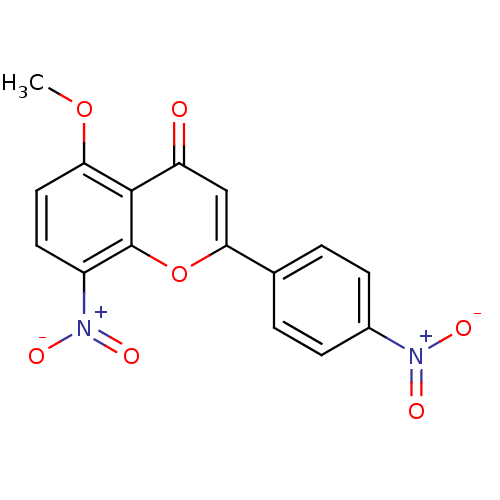 (5-Methoxy-8-nitro-2-(4-nitro-phenyl)-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037403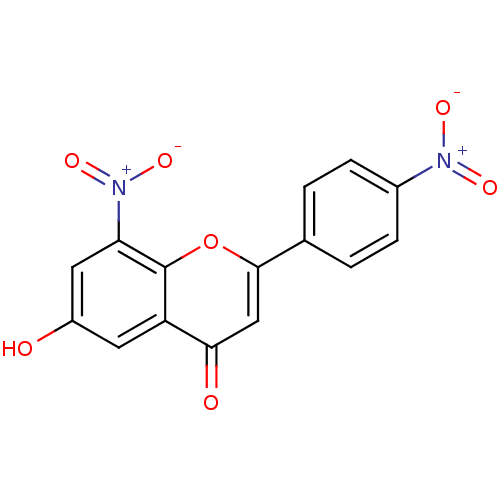 (6-Hydroxy-8-nitro-2-(4-nitro-phenyl)-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037415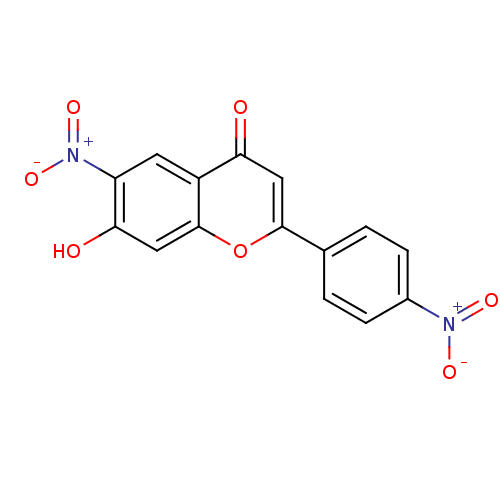 (7-Hydroxy-6-nitro-2-(4-nitro-phenyl)-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037404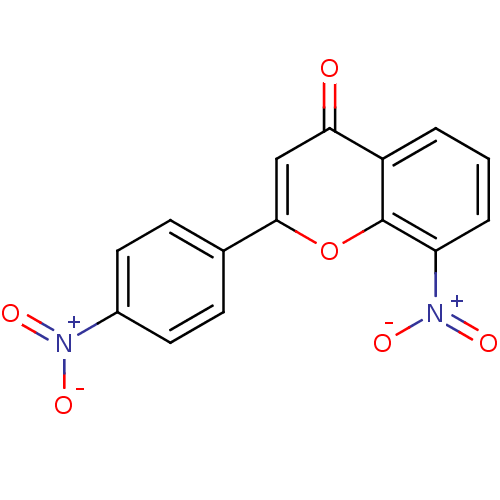 (8-Nitro-2-(4-nitro-phenyl)-chromen-4-one | CHEMBL3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037400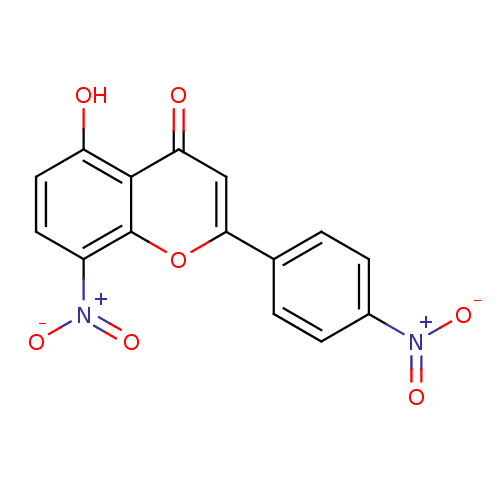 (5-Hydroxy-8-nitro-2-(4-nitro-phenyl)-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50037416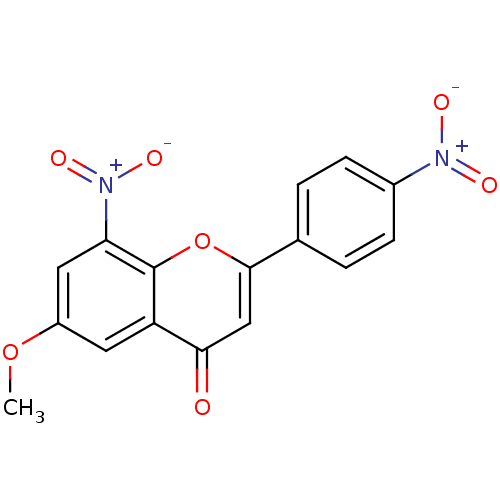 (6-Methoxy-8-nitro-2-(4-nitro-phenyl)-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description In vitro inhibition of protein-tyrosine activity of p56lck enzyme in presence of 50 uM ATP | J Med Chem 37: 3353-62 (1994) BindingDB Entry DOI: 10.7270/Q2D21WNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||