

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041043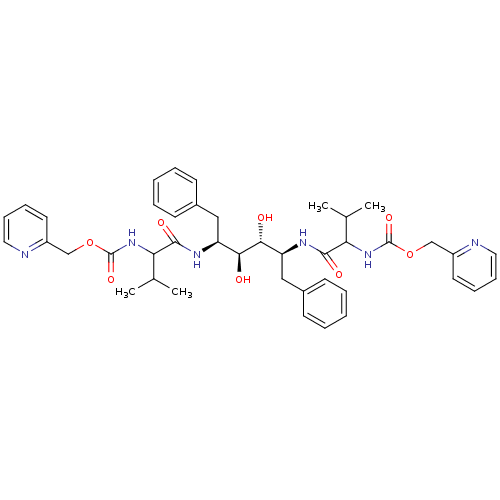 ((1-{(1S,2S,3R,4S)-1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041045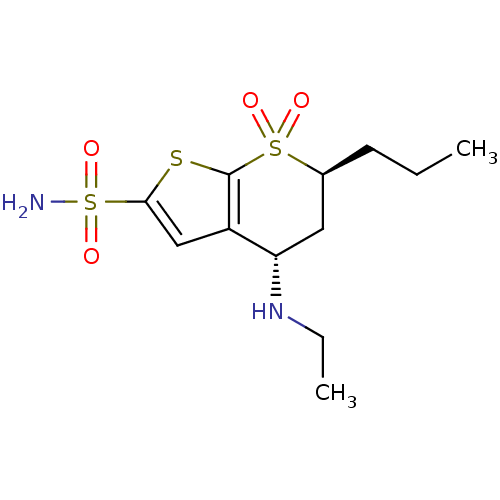 ((4S,6S)-4-Ethylamino-7,7-dioxo-6-propyl-4,5,6,7-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM197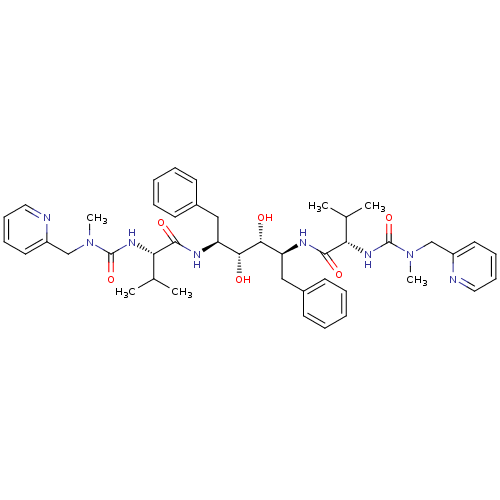 ((2S)-N-[(2S,3R,4R,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041023 ((1-{(1S,2S,3S,4S)-1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041037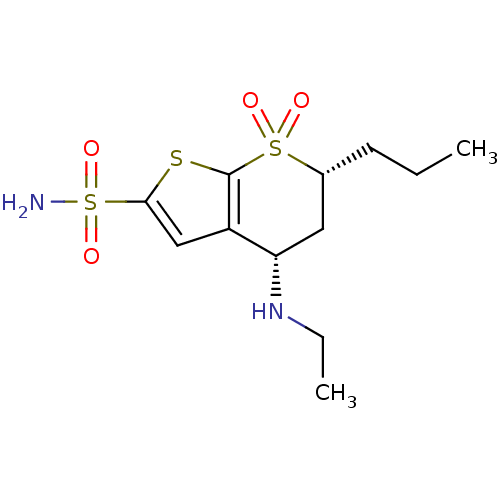 ((4S,6R)-4-Ethylamino-7,7-dioxo-6-propyl-4,5,6,7-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041035 (CHEMBL269401 | N-{(1S,2R,3R,4S)-1-Benzyl-2,3-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041032 ((1-{(1S,2R,3R,4S)-1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041027 ((4S,6S)-6-Ethyl-4-ethylamino-7,7-dioxo-4,5,6,7-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041029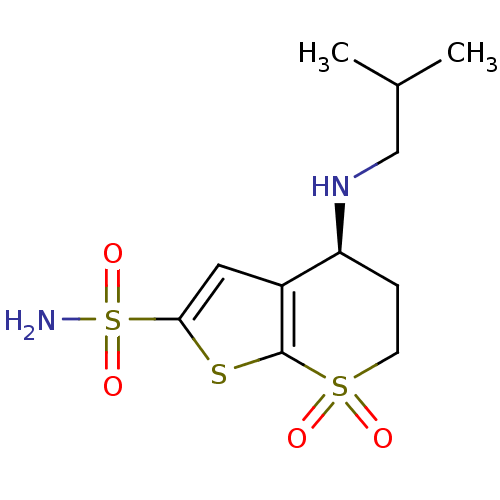 ((S)-4-Isobutylamino-7,7-dioxo-4,5,6,7-tetrahydro-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the affinity towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041048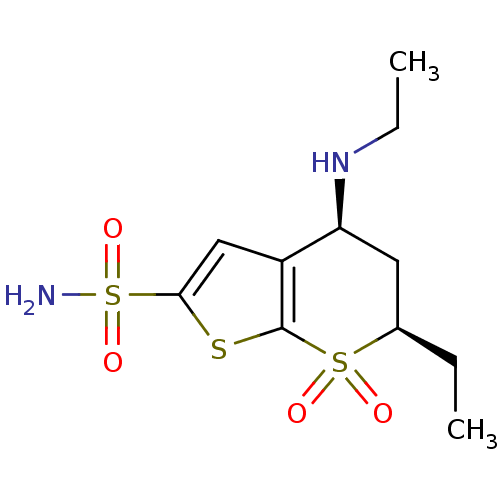 ((4S,6R)-6-Ethyl-4-ethylamino-7,7-dioxo-4,5,6,7-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041033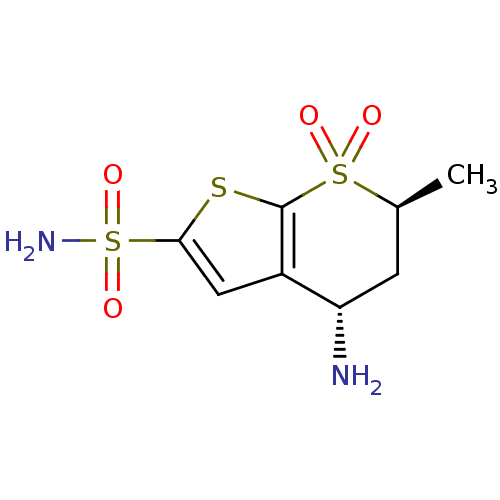 ((4S,6S)-4-Amino-6-methyl-7,7-dioxo-4,5,6,7-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041026 (CHEMBL10113 | N-{(1S,2S,3S,4S)-1-Benzyl-2,3-dihydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041041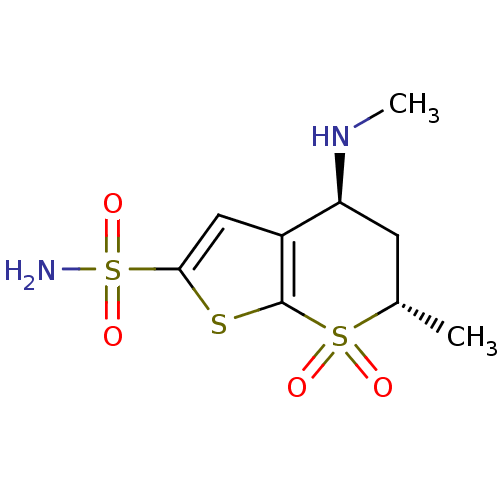 ((4S,6S)-6-Methyl-4-methylamino-7,7-dioxo-4,5,6,7-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the affinity towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005186 ((1,5-Dimethyl-2-methylimino-1,2-dihydro-benzo[cd]i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant of human thymidylate synthase | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005210 ((2-Imino-1-methyl-1,2-dihydro-benzo[cd]indol-6-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant of human thymidylate synthase | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041042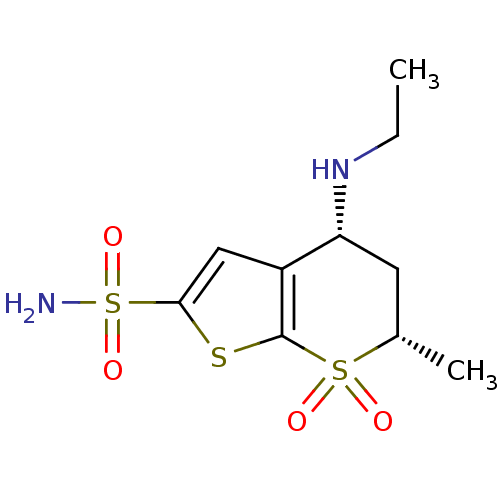 ((4R,6S)-4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the affinity towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM200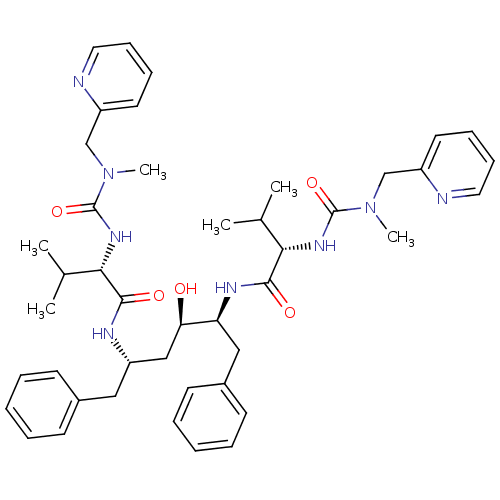 ((2S)-N-[(2S,3R,5S)-3-hydroxy-5-[(2S)-3-methyl-2-{[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005203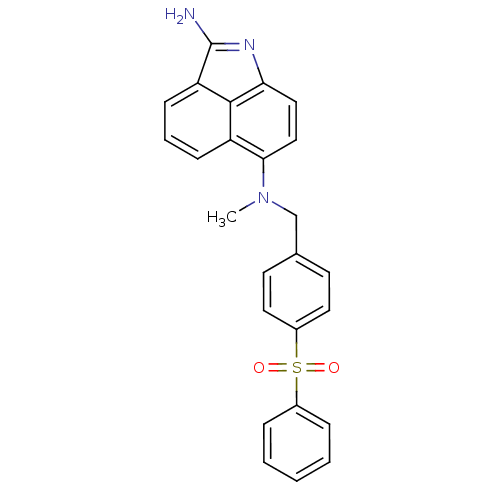 ((4-Benzenesulfonyl-benzyl)-(2-imino-1-methyl-1,2-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant of human thymidylate synthase | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041028 ((R)-4-Isobutylamino-7,7-dioxo-4,5,6,7-tetrahydro-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the affinity towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041038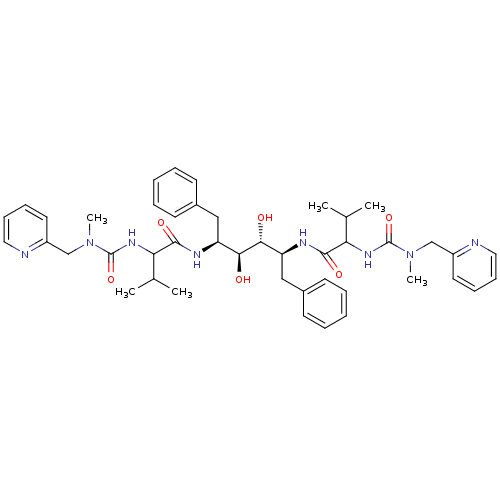 ((S)-N-{(1S,2R,3S,4S)-1-Benzyl-2,3-dihydroxy-4-[(S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant towards Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005190 (6-{Ethyl-[4-(piperazine-1-sulfonyl)-benzyl]-amino}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory constant of human thymidylate synthase | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041040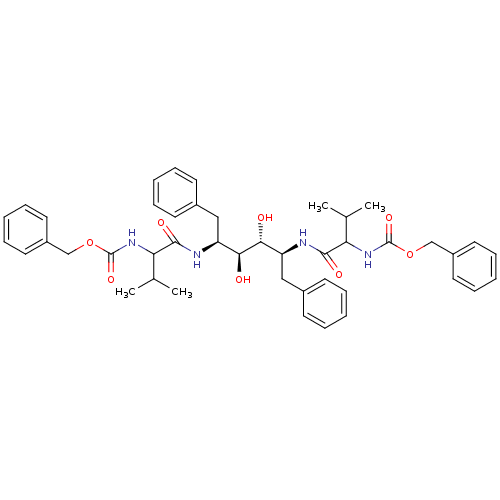 (CHEMBL9705 | {1-[(1S,2S,3R,4S)-1-Benzyl-4-(2-benzy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041034 (CHEMBL9544 | {1-[(1S,2S,3S,4S)-1-Benzyl-4-(2-benzy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041030 (CHEMBL429722 | {1-[(1S,2R,3R,4S)-1-Benzyl-4-(2-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041029 ((S)-4-Isobutylamino-7,7-dioxo-4,5,6,7-tetrahydro-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041039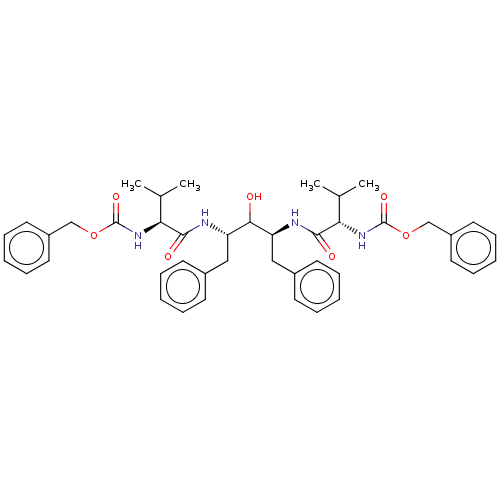 (CHEMBL307193 | {1-[(1S,3S)-1-Benzyl-3-(2-benzyloxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041046 (2-Acetylamino-N-(1-{(1S,3S)-3-[2-(2-acetylamino-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50421874 (CHEMBL2311105) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50009257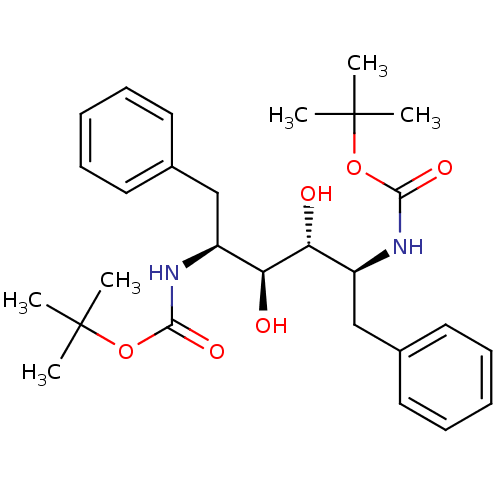 (((1S,2R,3S,4S)-1-Benzyl-4-tert-butoxycarbonylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041042 ((4R,6S)-4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014153 (((1S,2S,3S,4S)-1-Benzyl-4-tert-butoxycarbonylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041028 ((R)-4-Isobutylamino-7,7-dioxo-4,5,6,7-tetrahydro-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014151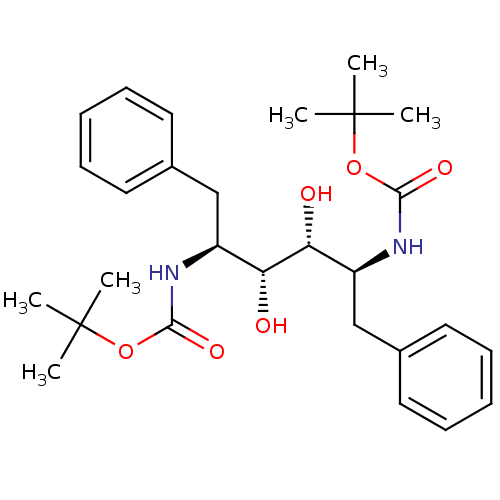 (((1S,2R,3R,4S)-1-Benzyl-4-tert-butoxycarbonylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041047 (2-Amino-N-[(1S,3S)-3-(2-amino-3-methyl-butyrylamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041036 (((1S,3S)-1-Benzyl-3-tert-butoxycarbonylamino-2-hyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50009244 (CHEMBL9735 | N-((1S,3S)-3-Acetylamino-1-benzyl-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041044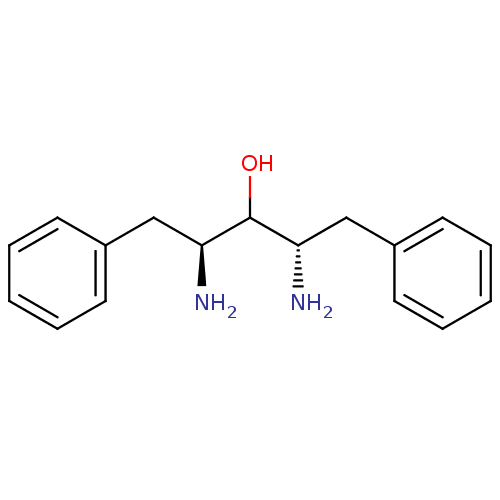 ((2S,4S)-2,4-Diamino-1,5-diphenyl-pentan-3-ol | 2,4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid UniChem Patents Similars | MMDB PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041026 (CHEMBL10113 | N-{(1S,2S,3S,4S)-1-Benzyl-2,3-dihydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Effective concentration of compound for inhibition of HIV 1 protease was determined | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041023 ((1-{(1S,2S,3S,4S)-1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Effective concentration of compound for inhibition of HIV 1 protease was determined | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041035 (CHEMBL269401 | N-{(1S,2R,3R,4S)-1-Benzyl-2,3-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Effective concentration of compound for inhibition of HIV 1 protease was determined | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041043 ((1-{(1S,2S,3R,4S)-1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Effective concentration of compound for inhibition of HIV 1 protease was determined | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50041032 ((1-{(1S,2R,3R,4S)-1-Benzyl-2,3-dihydroxy-4-[3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Effective concentration of compound for inhibition of HIV 1 protease was determined | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM197 ((2S)-N-[(2S,3R,4R,5S)-3,4-dihydroxy-5-[(2S)-3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Effective concentration of compound for inhibition of HIV 1 protease was determined | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||