

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50450592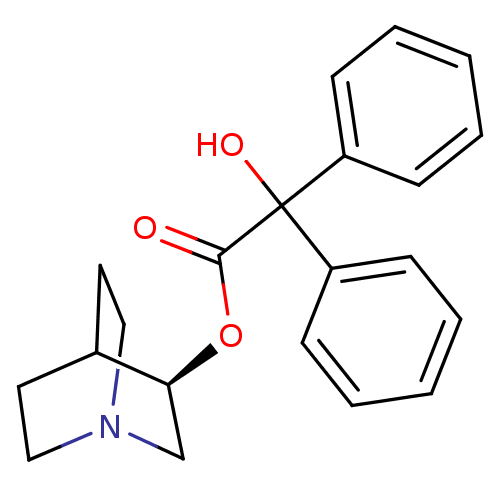 (CHEMBL558910) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory Curated by ChEMBL | Assay Description Ability to displace [3H](-)-quinuclidinyl bezilate(QNB) from M2 receptor in rat heart homogenate | J Med Chem 36: 848-54 (1993) BindingDB Entry DOI: 10.7270/Q2S46SM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50403547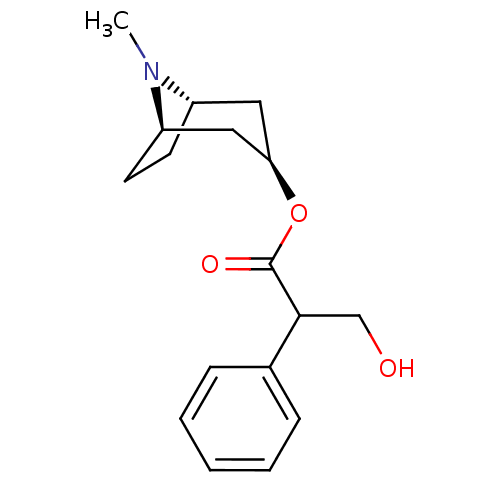 (ATROPEN | ATROPINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory Curated by ChEMBL | Assay Description Ability to displace [3H]N-methylscopolamine (NMS) from M3 receptor in rat submaxillary gland homogenate | J Med Chem 36: 848-54 (1993) BindingDB Entry DOI: 10.7270/Q2S46SM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50450592 (CHEMBL558910) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory Curated by ChEMBL | Assay Description Ability to displace [3H]pirenzepine (PZ) from M1 receptor in rat cortex homogenate | J Med Chem 36: 848-54 (1993) BindingDB Entry DOI: 10.7270/Q2S46SM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50450592 (CHEMBL558910) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory Curated by ChEMBL | Assay Description Ability to displace [3H]N-methylscopolamine (NMS) from M3 receptor in rat submaxillary gland homogenate | J Med Chem 36: 848-54 (1993) BindingDB Entry DOI: 10.7270/Q2S46SM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50403547 (ATROPEN | ATROPINE) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory Curated by ChEMBL | Assay Description Ability to displace [3H]pirenzepine (PZ) from M1 receptor in rat cortex homogenate | J Med Chem 36: 848-54 (1993) BindingDB Entry DOI: 10.7270/Q2S46SM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50456344 (CHEMBL2112942) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory Curated by ChEMBL | Assay Description Ability to displace [3H]N-methylscopolamine (NMS) from M3 receptor in rat submaxillary gland homogenate | J Med Chem 36: 848-54 (1993) BindingDB Entry DOI: 10.7270/Q2S46SM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50403547 (ATROPEN | ATROPINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory Curated by ChEMBL | Assay Description Ability to displace [3H](-)-quinuclidinyl bezilate(QNB) from M2 receptor in rat heart homogenate | J Med Chem 36: 848-54 (1993) BindingDB Entry DOI: 10.7270/Q2S46SM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50456344 (CHEMBL2112942) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory Curated by ChEMBL | Assay Description Ability to displace [3H]pirenzepine (PZ) from M1 receptor in rat cortex homogenate | J Med Chem 36: 848-54 (1993) BindingDB Entry DOI: 10.7270/Q2S46SM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50456344 (CHEMBL2112942) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory Curated by ChEMBL | Assay Description Ability to displace [3H](-)-quinuclidinyl bezilate(QNB) from M2 receptor in rat heart homogenate | J Med Chem 36: 848-54 (1993) BindingDB Entry DOI: 10.7270/Q2S46SM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM39341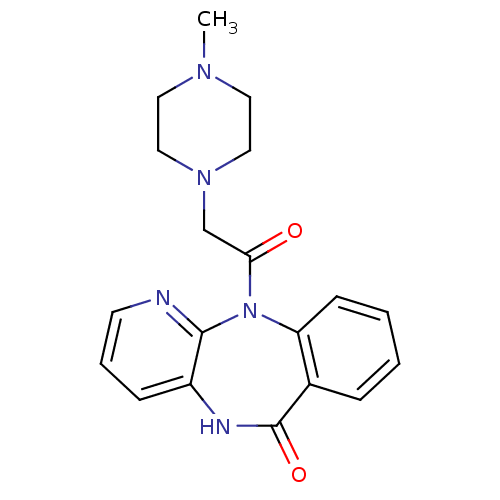 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory Curated by ChEMBL | Assay Description Ability to displace [3H]pirenzepine (PZ) from M1 receptor in rat cortex homogenate | J Med Chem 36: 848-54 (1993) BindingDB Entry DOI: 10.7270/Q2S46SM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory Curated by ChEMBL | Assay Description Ability to displace [3H]N-methylscopolamine (NMS) from M3 receptor in rat submaxillary gland homogenate | J Med Chem 36: 848-54 (1993) BindingDB Entry DOI: 10.7270/Q2S46SM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM39341 (11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oak Ridge National Laboratory Curated by ChEMBL | Assay Description Ability to displace [3H](-)-quinuclidinyl bezilate(QNB) from M2 receptor in rat heart homogenate | J Med Chem 36: 848-54 (1993) BindingDB Entry DOI: 10.7270/Q2S46SM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||