

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L-selectin (Homo sapiens (Human)) | BDBM50050461 (5-(2-{2-[5-Acetylamino-2-hydroxymethyl-4-(2,3,4-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric L-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50050461 (5-(2-{2-[5-Acetylamino-2-hydroxymethyl-4-(2,3,4-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric Selectin P-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-selectin (Homo sapiens (Human)) | BDBM50050456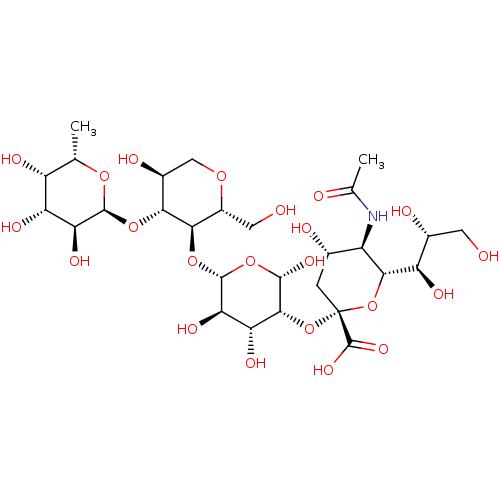 (5-(2-{3,5-Dihydroxy-2-hydroxymethyl-6-[2-hydroxyme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric L-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50050456 (5-(2-{3,5-Dihydroxy-2-hydroxymethyl-6-[2-hydroxyme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric E-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50050458 (5-Acetylamino-2-{3,5-dihydroxy-2-hydroxymethyl-6-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric E-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-selectin (Homo sapiens (Human)) | BDBM50050460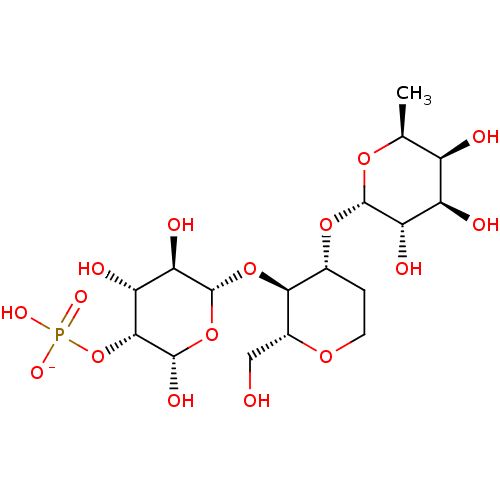 (CHEMBL175724 | sodium salt of 6-[2-Hydroxy-4-(2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric L-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50050455 (CHEMBL177316 | sodium salt of Sulfuric acid mono-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric Selectin P-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50450369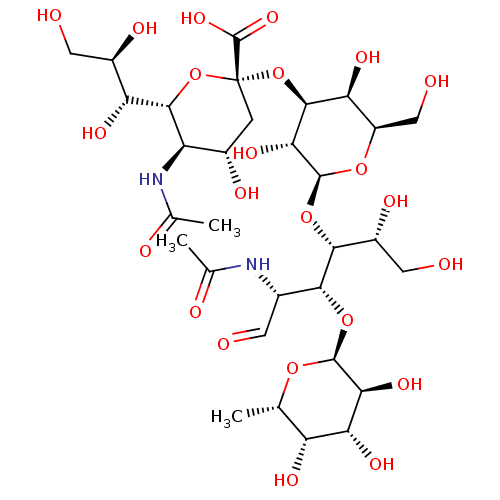 (SIALYL LEWIS X | Sialyl LeX | Sialyl lewis-x | sLe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric E-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50050459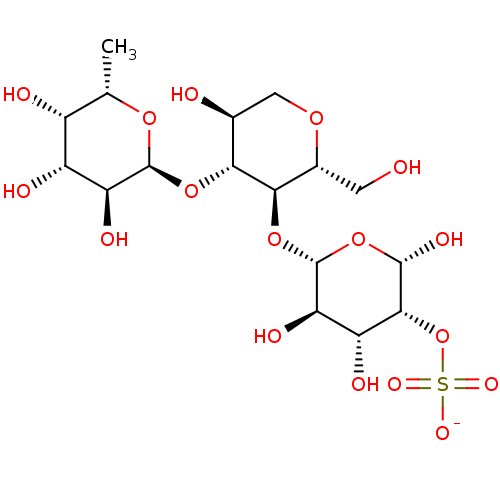 (CHEMBL362419 | O-(3-O-Sulfo-beta-D-galactopyranosy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric Selectin P-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-selectin (Homo sapiens (Human)) | BDBM50050455 (CHEMBL177316 | sodium salt of Sulfuric acid mono-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric L-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50050461 (5-(2-{2-[5-Acetylamino-2-hydroxymethyl-4-(2,3,4-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric E-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-selectin (Homo sapiens (Human)) | BDBM50050458 (5-Acetylamino-2-{3,5-dihydroxy-2-hydroxymethyl-6-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric L-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50050459 (CHEMBL362419 | O-(3-O-Sulfo-beta-D-galactopyranosy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric E-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50450369 (SIALYL LEWIS X | Sialyl LeX | Sialyl lewis-x | sLe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric Selectin P-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50050458 (5-Acetylamino-2-{3,5-dihydroxy-2-hydroxymethyl-6-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric Selectin P-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50050460 (CHEMBL175724 | sodium salt of 6-[2-Hydroxy-4-(2,3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric Selectin P-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50050455 (CHEMBL177316 | sodium salt of Sulfuric acid mono-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric E-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-selectin (Homo sapiens (Human)) | BDBM50450369 (SIALYL LEWIS X | Sialyl LeX | Sialyl lewis-x | sLe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric L-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50050454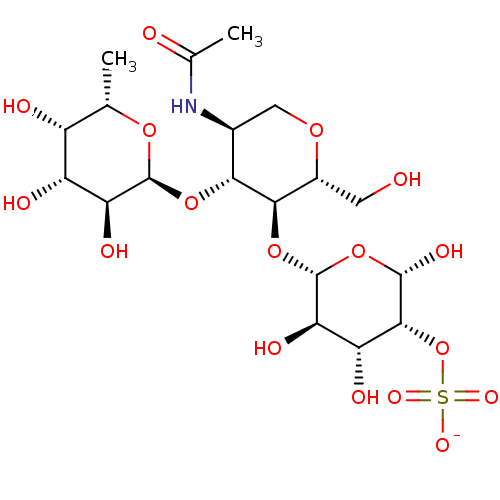 (CHEMBL177789 | O-(3-O-Sulfo-beta-D-galactopyranosy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric Selectin P-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-selectin (Homo sapiens (Human)) | BDBM50050456 (5-(2-{3,5-Dihydroxy-2-hydroxymethyl-6-[2-hydroxyme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric Selectin P-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50050454 (CHEMBL177789 | O-(3-O-Sulfo-beta-D-galactopyranosy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric E-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-selectin (Homo sapiens (Human)) | BDBM50050454 (CHEMBL177789 | O-(3-O-Sulfo-beta-D-galactopyranosy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric L-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-selectin (Homo sapiens (Human)) | BDBM50050459 (CHEMBL362419 | O-(3-O-Sulfo-beta-D-galactopyranosy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric L-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E-selectin (Homo sapiens (Human)) | BDBM50050460 (CHEMBL175724 | sodium salt of 6-[2-Hydroxy-4-(2,3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human chimeric E-selectin-Ig | J Med Chem 39: 1339-43 (1996) Article DOI: 10.1021/jm9506478 BindingDB Entry DOI: 10.7270/Q2513ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||