

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50041291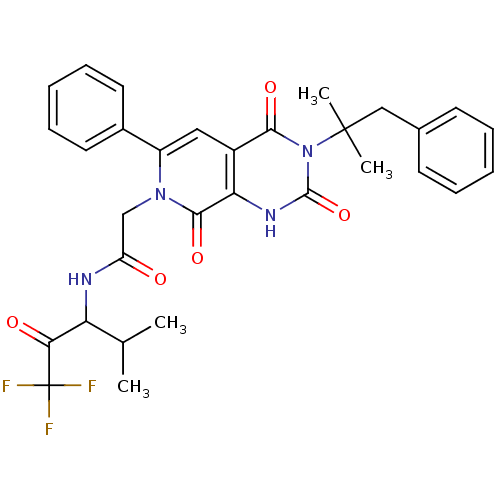 (2-[(R)-3-(1,1-Dimethyl-2-phenyl-ethyl)-2,4,8-triox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036058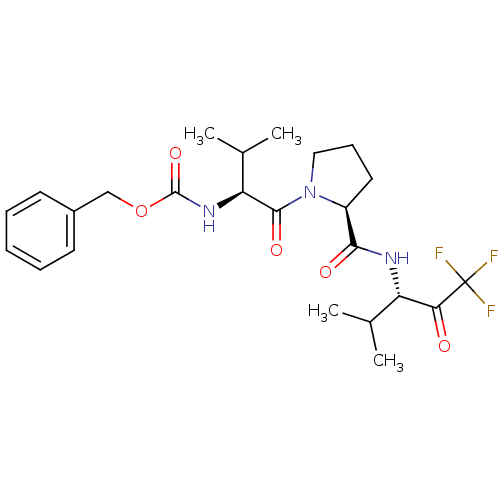 (CHEMBL354883 | benzyl (S)-1-((S)-2-(((S)-1,1,1-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051162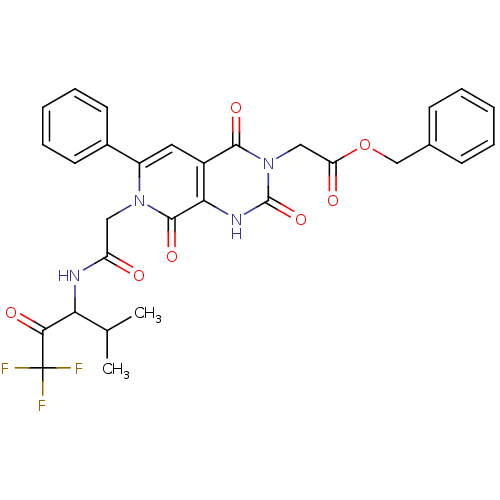 (CHEMBL13753 | {(R)-2,4,8-Trioxo-6-phenyl-7-[(3,3,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036127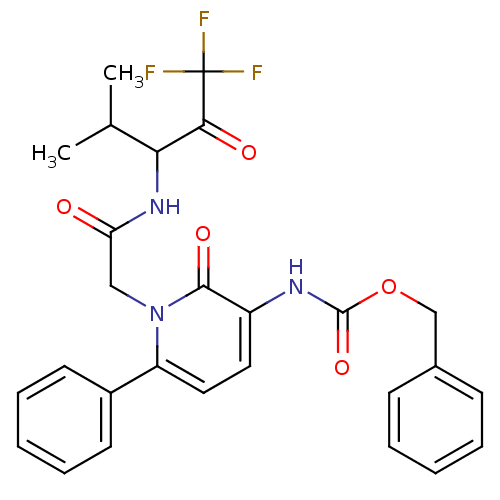 (CHEMBL11391 | {2-Oxo-6-phenyl-1-[(3,3,3-trifluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036127 (CHEMBL11391 | {2-Oxo-6-phenyl-1-[(3,3,3-trifluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051160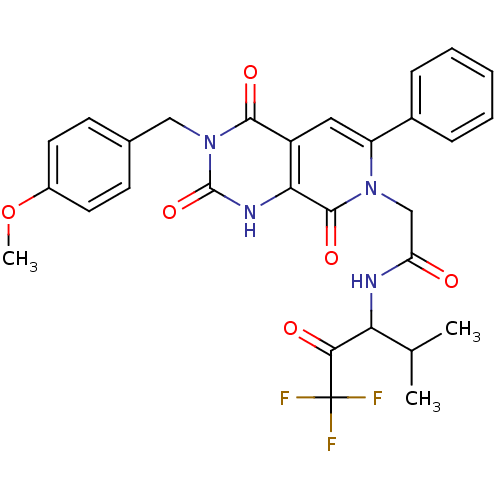 (2-[(R)-3-(4-Methoxy-benzyl)-2,4,8-trioxo-6-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051147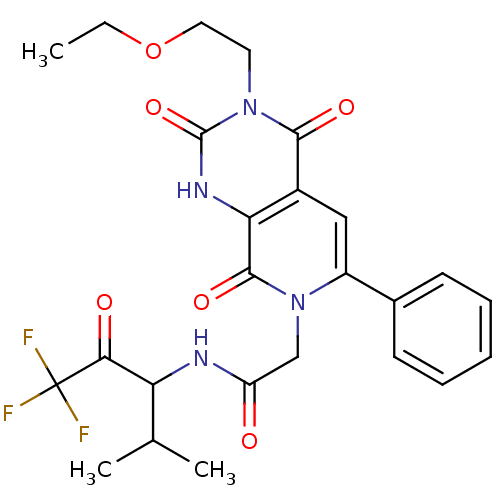 (2-[(R)-3-(2-Ethoxy-ethyl)-2,4,8-trioxo-6-phenyl-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50037375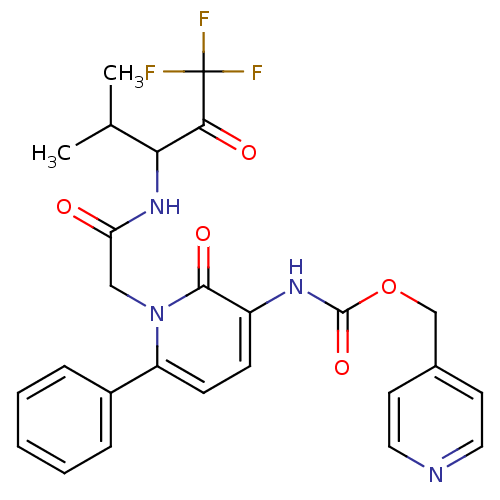 (CHEMBL275043 | {2-Oxo-6-phenyl-1-[(3,3,3-trifluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051143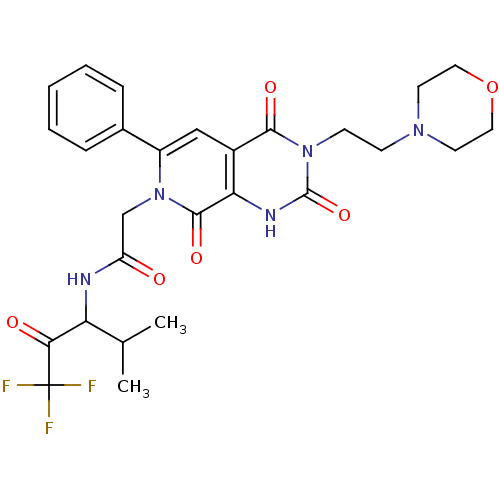 (2-[(R)-3-(2-Morpholin-4-yl-ethyl)-2,4,8-trioxo-6-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051137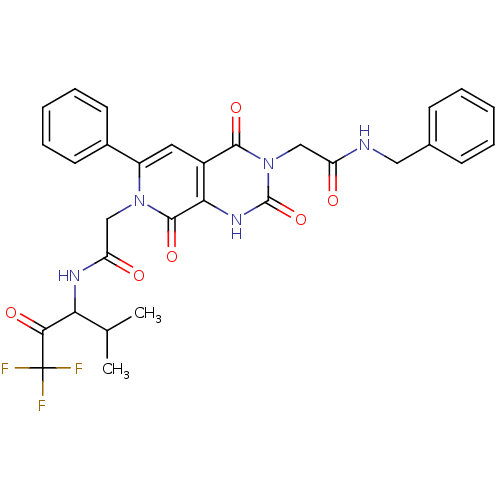 (2-[(R)-3-(Benzylcarbamoyl-methyl)-2,4,8-trioxo-6-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051156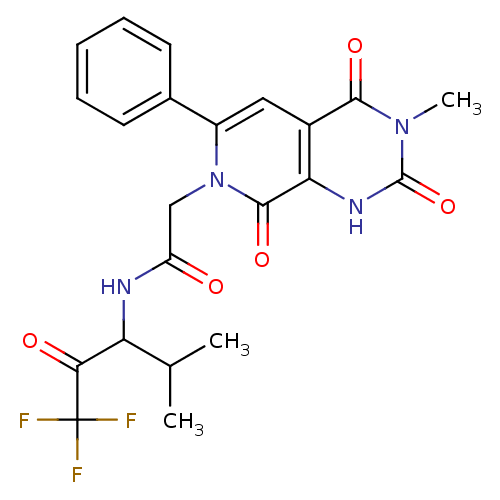 (2-((R)-3-Methyl-2,4,8-trioxo-6-phenyl-2,3,4,8-tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051158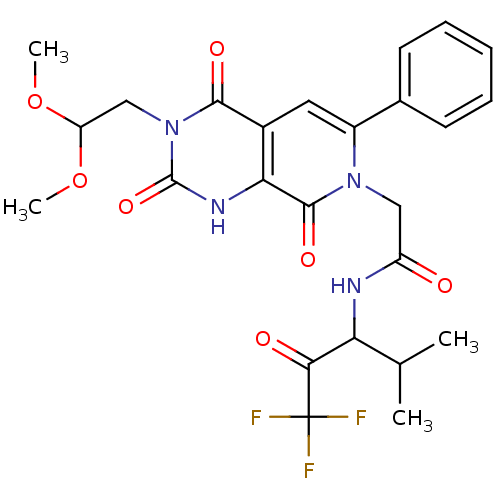 (2-[(R)-3-(2,2-Dimethoxy-ethyl)-2,4,8-trioxo-6-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051139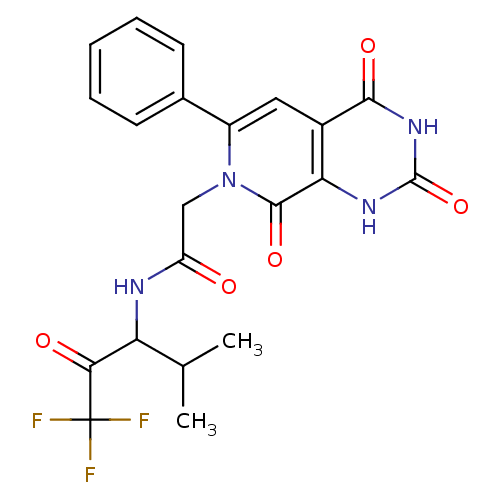 (CHEMBL13863 | N-(3,3,3-Trifluoro-1-isopropyl-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051150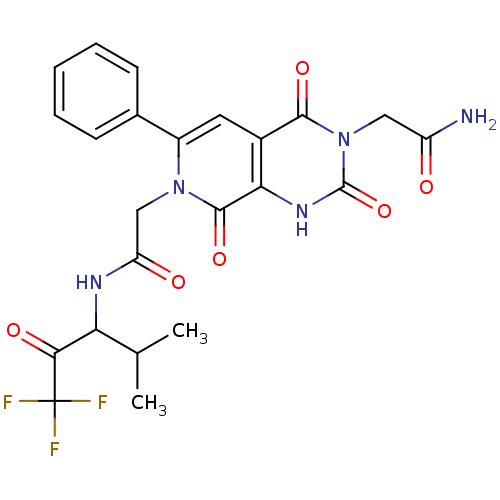 (2-{(R)-2,4,8-Trioxo-6-phenyl-7-[(3,3,3-trifluoro-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50037352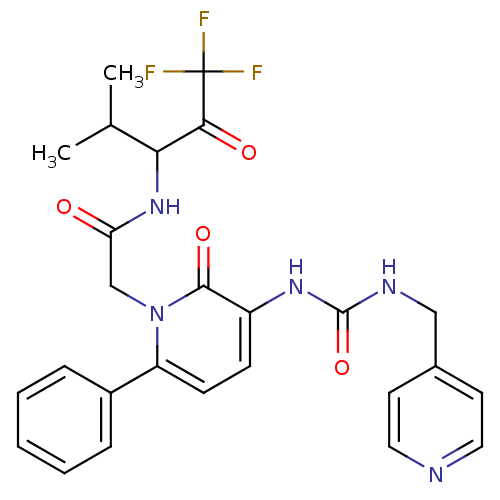 (2-[2-Oxo-6-phenyl-3-((S)-3-pyridin-4-ylmethyl-urei...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051155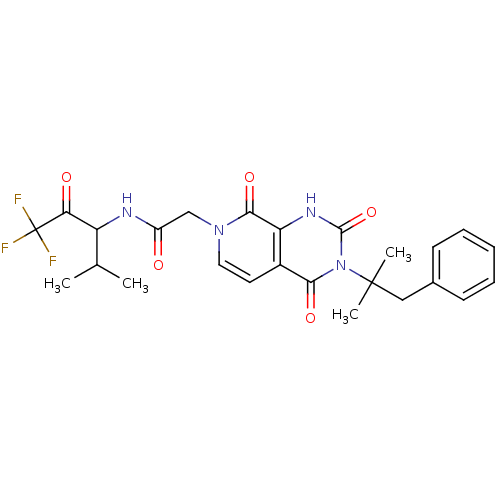 (2-[(R)-3-(1,1-Dimethyl-2-phenyl-ethyl)-2,4,8-triox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051159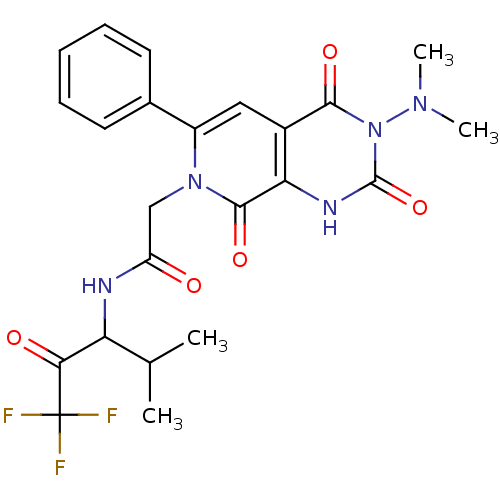 (2-((R)-3-Dimethylamino-2,4,8-trioxo-6-phenyl-2,3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051144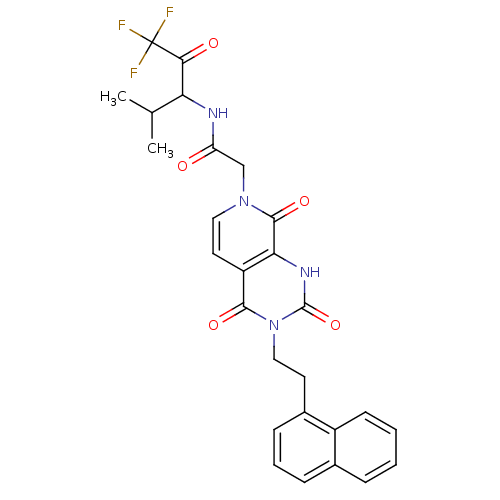 (2-[(R)-3-(2-Naphthalen-1-yl-ethyl)-2,4,8-trioxo-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051152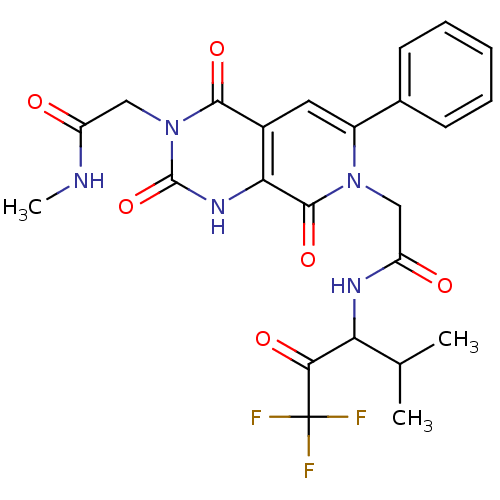 (2-((R)-3-Methylcarbamoylmethyl-2,4,8-trioxo-6-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051135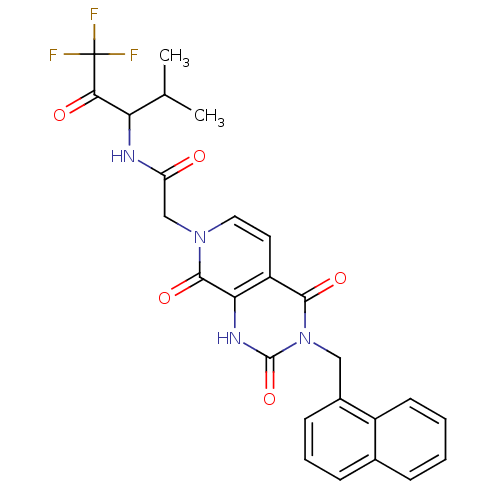 (2-((R)-3-Naphthalen-1-ylmethyl-2,4,8-trioxo-2,3,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051141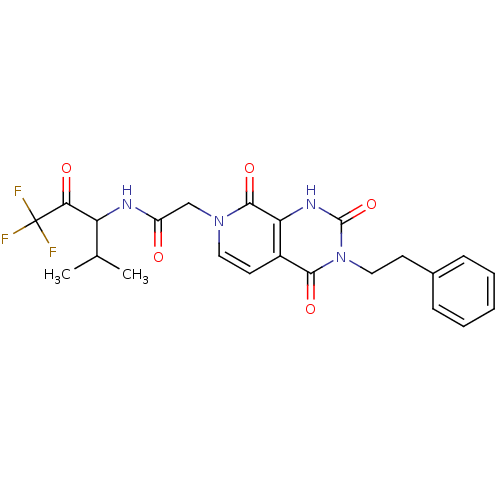 (CHEMBL14064 | N-(3,3,3-Trifluoro-1-isopropyl-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051136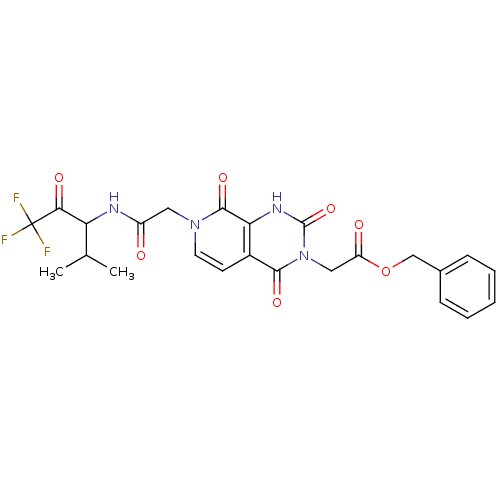 (CHEMBL13740 | {(R)-2,4,8-Trioxo-7-[(3,3,3-trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50037346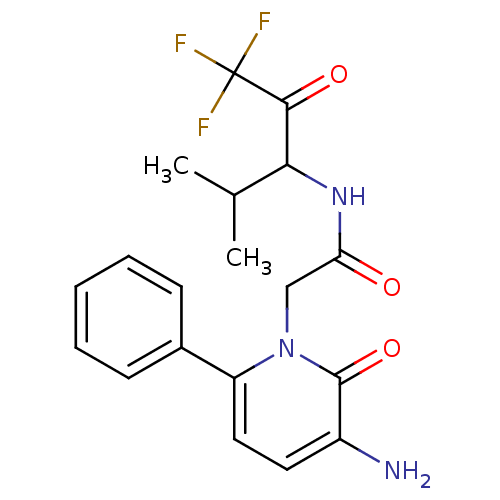 (2-(3-Amino-2-oxo-6-phenyl-2H-pyridin-1-yl)-N-(3,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051148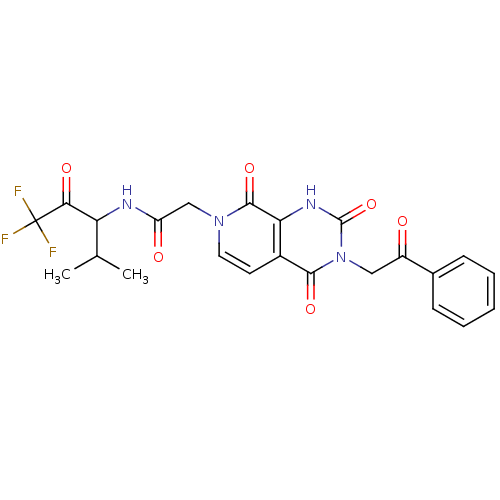 (CHEMBL14222 | N-(3,3,3-Trifluoro-1-isopropyl-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051138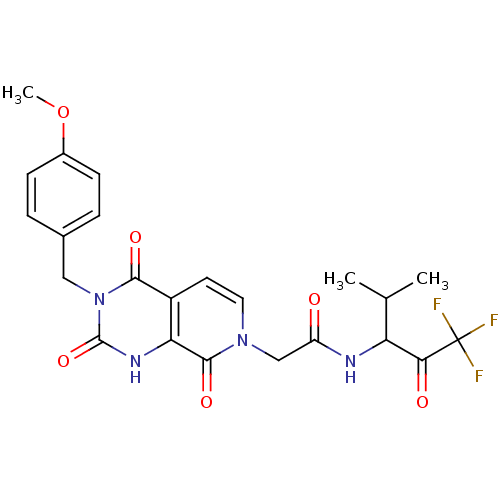 (2-[(R)-3-(4-Methoxy-benzyl)-2,4,8-trioxo-2,3,4,8-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051149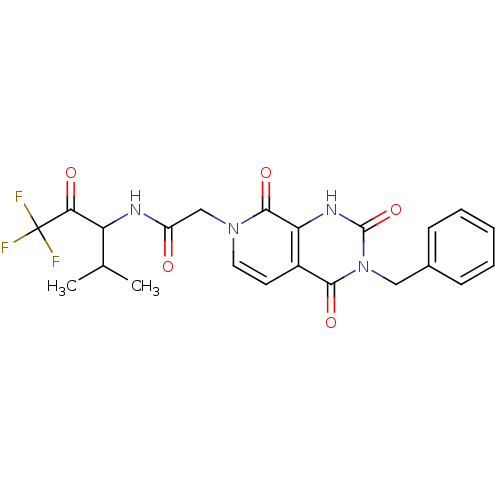 (2-((R)-3-Benzyl-2,4,8-trioxo-2,3,4,8-tetrahydro-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051161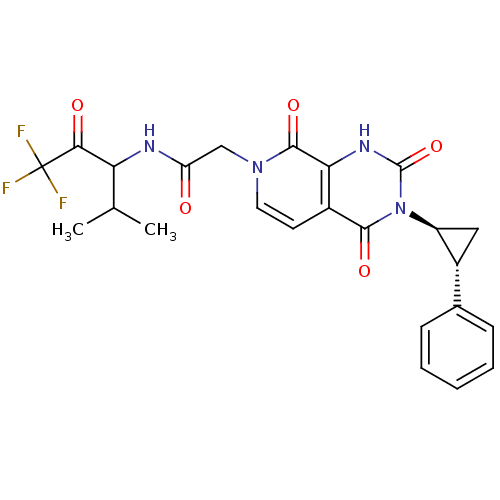 (CHEMBL276181 | N-(3,3,3-Trifluoro-1-isopropyl-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50037109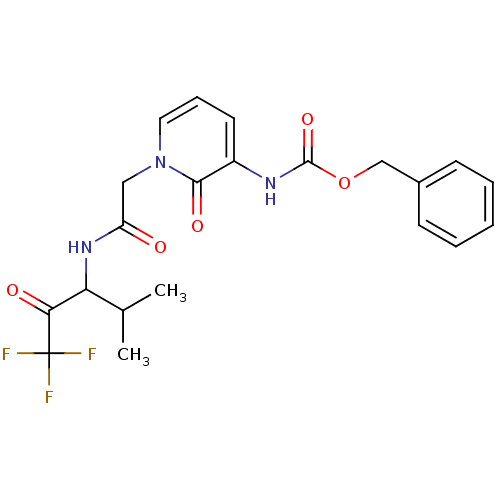 (CHEMBL13684 | {2-Oxo-1-[(3,3,3-trifluoro-1-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50037109 (CHEMBL13684 | {2-Oxo-1-[(3,3,3-trifluoro-1-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051154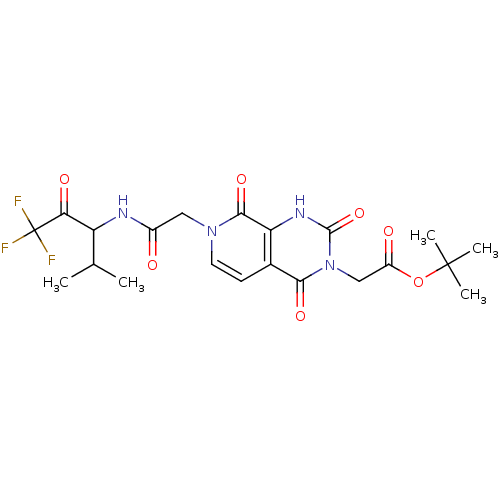 (CHEMBL14176 | {(R)-2,4,8-Trioxo-7-[(3,3,3-trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051142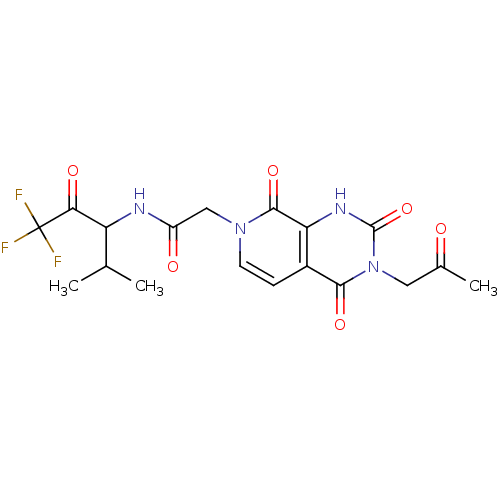 (CHEMBL13804 | N-(3,3,3-Trifluoro-1-isopropyl-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051140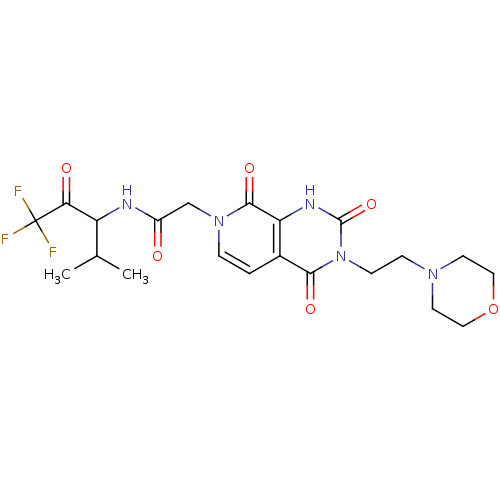 (2-[(R)-3-(2-Morpholin-4-yl-ethyl)-2,4,8-trioxo-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50037357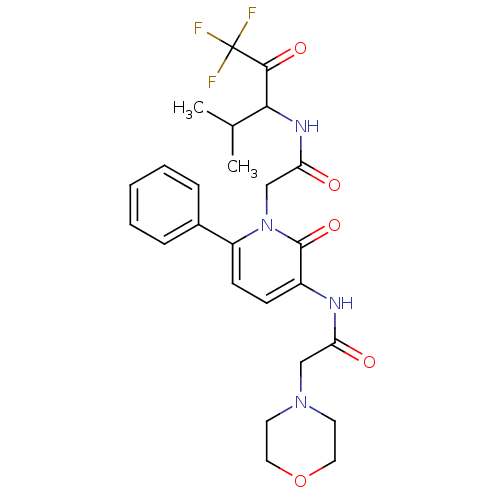 (2-[3-((S)-2-Morpholin-4-yl-acetylamino)-2-oxo-6-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 469 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051153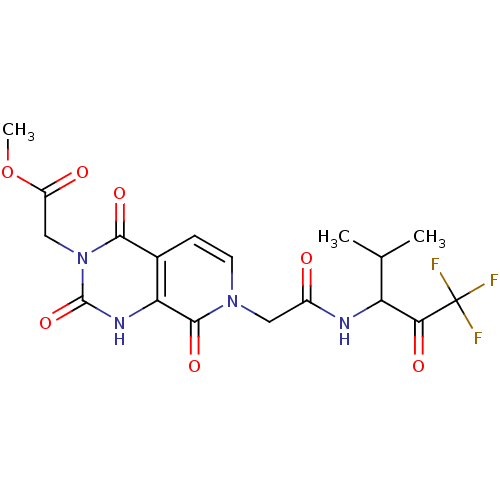 (CHEMBL14128 | {(R)-2,4,8-Trioxo-7-[(3,3,3-trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051145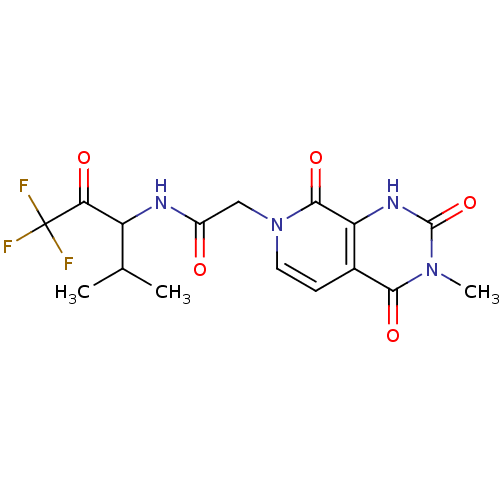 (2-((R)-3-Methyl-2,4,8-trioxo-2,3,4,8-tetrahydro-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051151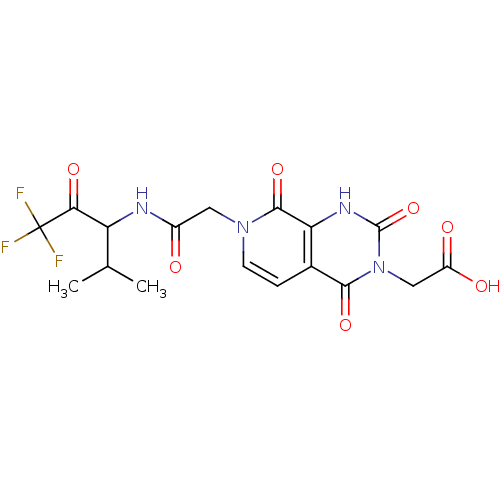 (CHEMBL273612 | {(R)-2,4,8-Trioxo-7-[(3,3,3-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051146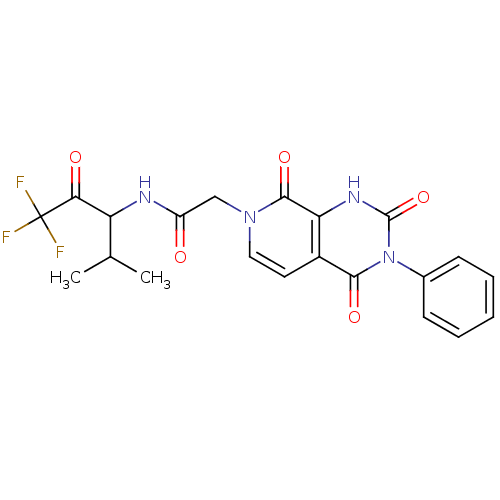 (CHEMBL276182 | N-(3,3,3-Trifluoro-1-isopropyl-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50051157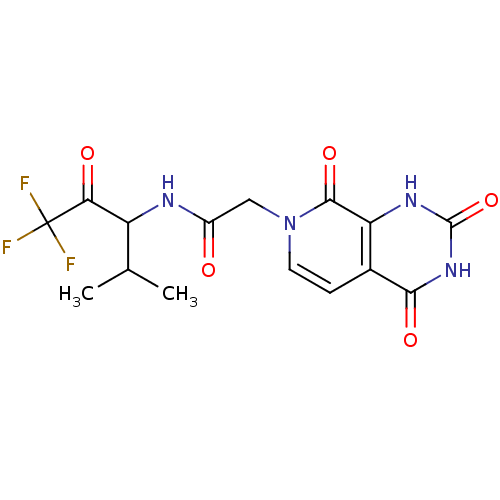 (CHEMBL275723 | N-(3,3,3-Trifluoro-1-isopropyl-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50037112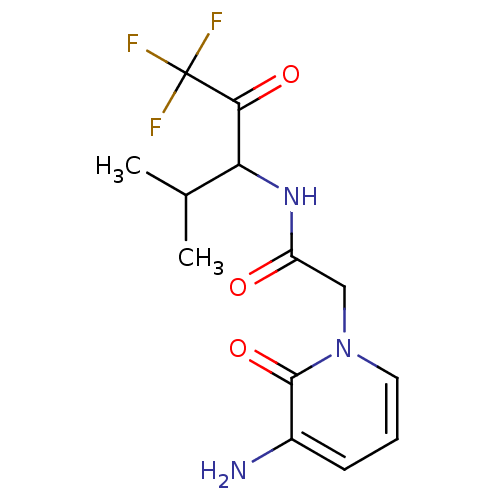 (2-(3-Amino-2-oxo-2H-pyridin-1-yl)-N-(3,3,3-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Human Neutrophil Elastase-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala-Ala-Pro-Val-p- nitroanilide. | J Med Chem 39: 1112-24 (1996) Article DOI: 10.1021/jm950684z BindingDB Entry DOI: 10.7270/Q25Q4V6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||