

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50225106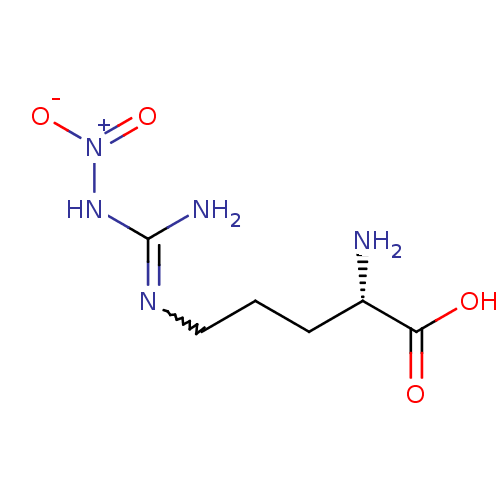 ((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against isomeric form of Neuronal nitric oxide synthase measured by citrulline assay | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50225106 ((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of conversion of radiolabeled arginine to citrulline by isomeric form of nitric oxide synthase Endothelial nitric oxide synthase ... | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065369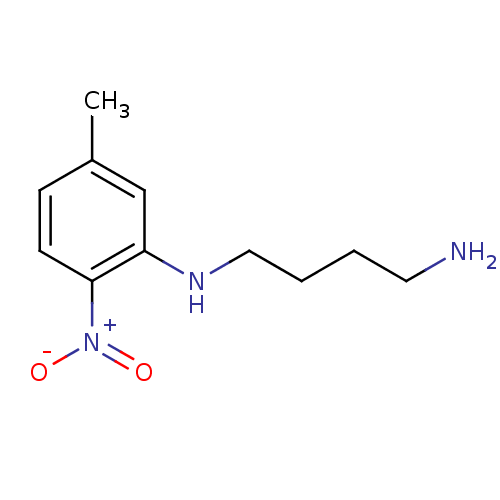 (CHEMBL89454 | N*1*-(5-Methyl-2-nitro-phenyl)-butan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065362 ((S)-2-Amino-5-(5-methyl-2-nitro-phenylamino)-penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065366 ((S)-2-Amino-5-(5-methyl-2-nitro-phenylamino)-penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065364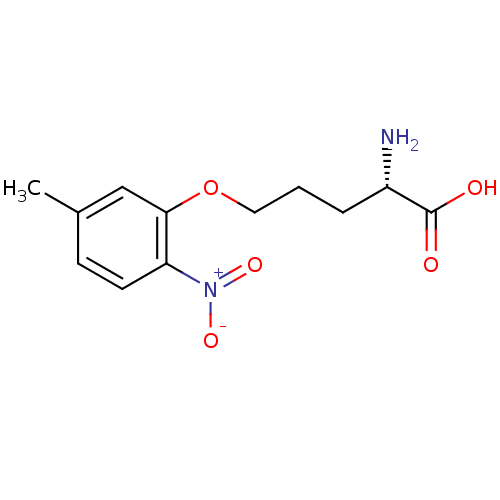 ((S)-2-Amino-5-(5-methyl-2-nitro-phenoxy)-pentanoic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065359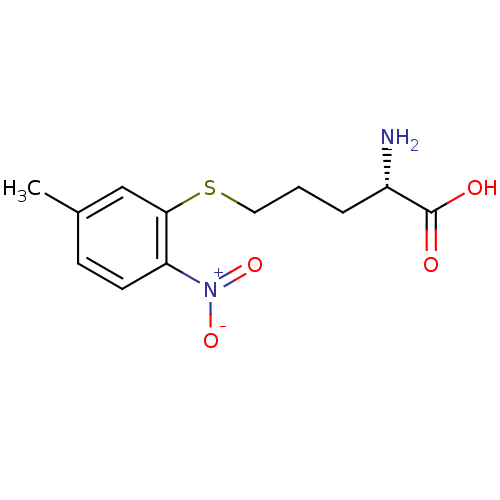 ((S)-2-Amino-5-(5-methyl-2-nitro-phenylsulfanyl)-pe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065359 ((S)-2-Amino-5-(5-methyl-2-nitro-phenylsulfanyl)-pe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50065362 ((S)-2-Amino-5-(5-methyl-2-nitro-phenylamino)-penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of conversion of radiolabeled arginine to citrulline by isomeric form of nitric oxide synthase Endothelial nitric oxide synthase ... | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065366 ((S)-2-Amino-5-(5-methyl-2-nitro-phenylamino)-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50230993 ((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of conversion of radiolabeled arginine to citrulline by isomeric form of nitric oxide synthase Endothelial nitric oxide synthase ... | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50230993 ((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against isomeric form of Neuronal nitric oxide synthase measured by citrulline assay | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50065359 ((S)-2-Amino-5-(5-methyl-2-nitro-phenylsulfanyl)-pe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of conversion of radiolabeled arginine to citrulline by isomeric form of nitric oxide synthase Endothelial nitric oxide synthase ... | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065369 (CHEMBL89454 | N*1*-(5-Methyl-2-nitro-phenyl)-butan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50065364 ((S)-2-Amino-5-(5-methyl-2-nitro-phenoxy)-pentanoic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of conversion of radiolabeled arginine to citrulline by isomeric form of nitric oxide synthase Endothelial nitric oxide synthase ... | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065362 ((S)-2-Amino-5-(5-methyl-2-nitro-phenylamino)-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50225106 ((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50065369 (CHEMBL89454 | N*1*-(5-Methyl-2-nitro-phenyl)-butan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of conversion of radiolabeled arginine to citrulline by isomeric form of nitric oxide synthase Endothelial nitric oxide synthase ... | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065371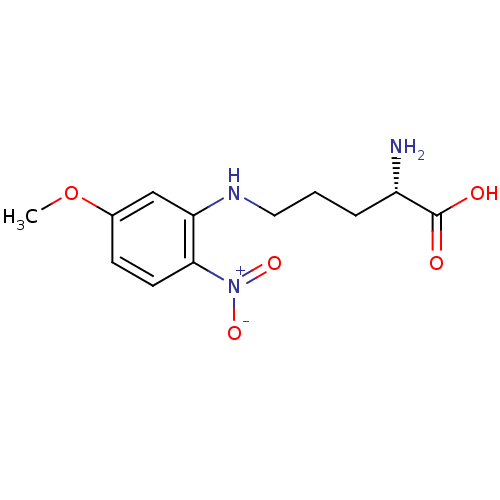 ((S)-2-Amino-5-(5-methoxy-2-nitro-phenylamino)-pent...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50230993 ((2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065375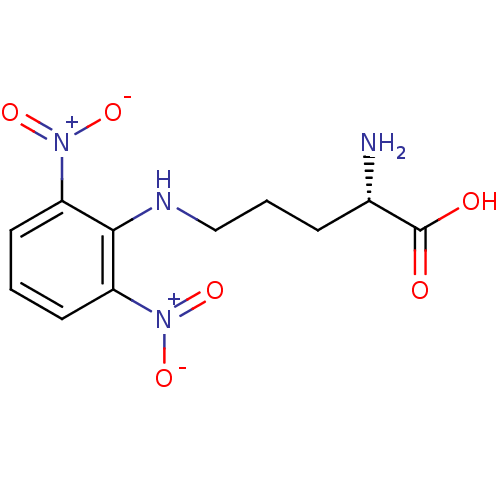 ((S)-2-Amino-5-(2,6-dinitro-phenylamino)-pentanoic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against isomeric form of Neuronal nitric oxide synthase measured by citrulline assay | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50065366 ((S)-2-Amino-5-(5-methyl-2-nitro-phenylamino)-penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of conversion of radiolabeled arginine to citrulline by isomeric form of nitric oxide synthase Endothelial nitric oxide synthase ... | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065374 ((S)-2-Amino-6-(5-methyl-2-nitro-phenylamino)-hexan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50065375 ((S)-2-Amino-5-(2,6-dinitro-phenylamino)-pentanoic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of conversion of radiolabeled arginine to citrulline by isomeric form of nitric oxide synthase Endothelial nitric oxide synthase ... | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065377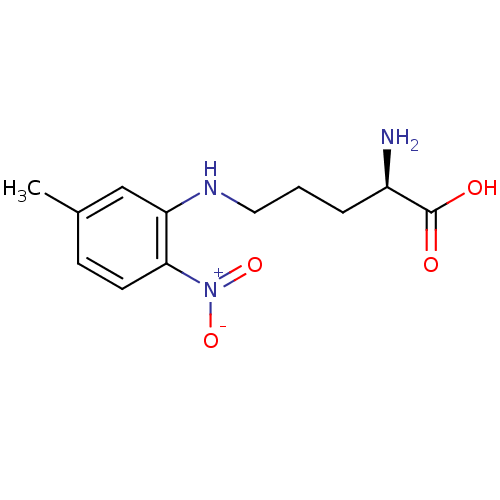 ((R)-2-Amino-5-(5-methyl-2-nitro-phenylamino)-penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065372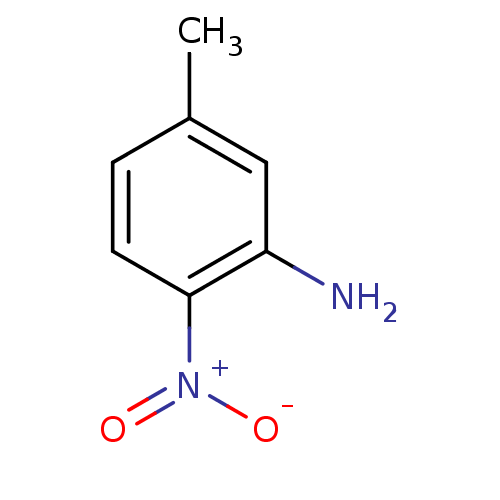 (5-Methyl-2-nitro-phenylamine | CHEMBL278016 | EN30...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against isomeric form of Neuronal nitric oxide synthase measured by citrulline assay | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065364 ((S)-2-Amino-5-(5-methyl-2-nitro-phenoxy)-pentanoic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065373 ((S)-2-Amino-5-(4-methyl-2-nitro-phenylamino)-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065363 ((S)-2-Amino-5-m-tolylamino-pentanoic acid | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065363 ((S)-2-Amino-5-m-tolylamino-pentanoic acid | CHEMBL...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065360 ((S)-2-Amino-5-(5-hydroxy-2-nitro-phenylamino)-pent...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065367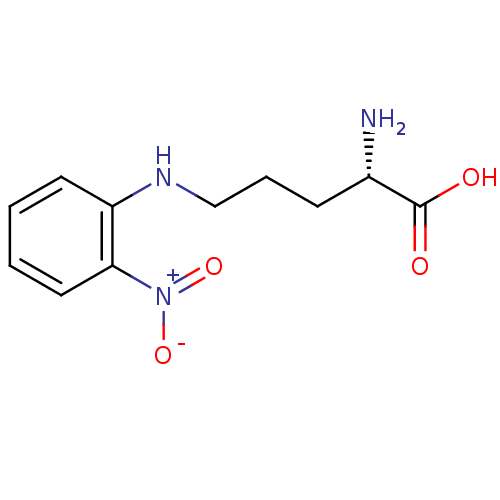 ((S)-2-Amino-5-(2-nitro-phenylamino)-pentanoic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065370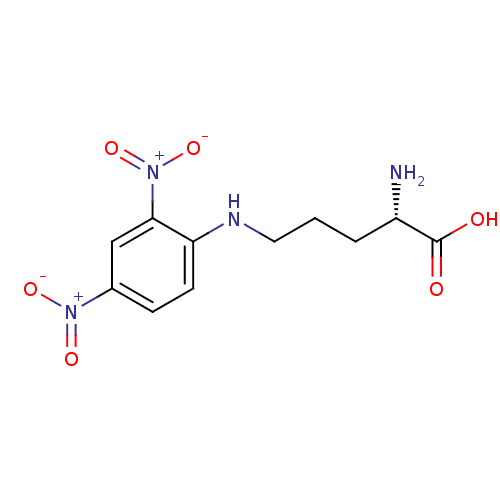 ((S)-2-Amino-5-(2,4-dinitro-phenylamino)-pentanoic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against isomeric form of Neuronal nitric oxide synthase measured by citrulline assay | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065365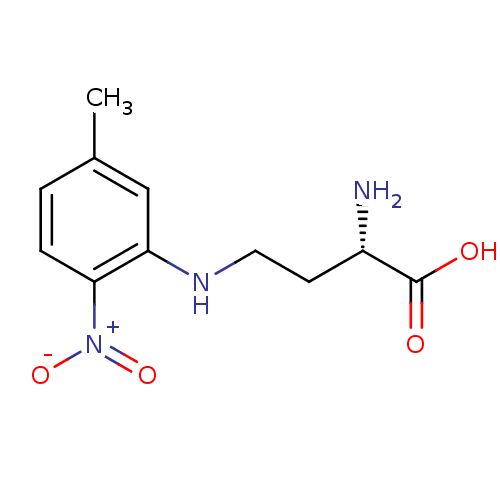 ((S)-2-Amino-4-(5-methyl-2-nitro-phenylamino)-butyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065376 (4-((S)-4-Amino-4-carboxy-butylamino)-3-nitro-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065376 (4-((S)-4-Amino-4-carboxy-butylamino)-3-nitro-benzo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against isomeric form of Neuronal nitric oxide synthase measured by citrulline assay | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065367 ((S)-2-Amino-5-(2-nitro-phenylamino)-pentanoic acid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against isomeric form of Neuronal nitric oxide synthase measured by citrulline assay | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065373 ((S)-2-Amino-5-(4-methyl-2-nitro-phenylamino)-penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against isomeric form of Neuronal nitric oxide synthase measured by citrulline assay | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065357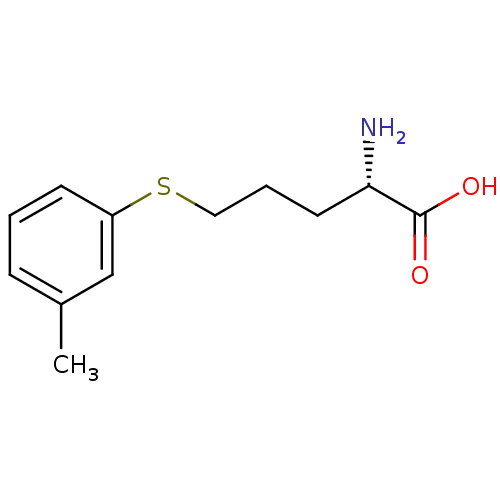 ((S)-2-Amino-5-m-tolylsulfanyl-pentanoic acid | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065368 ((S)-2-Amino-5-(2-nitro-4-trifluoromethyl-phenylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50065358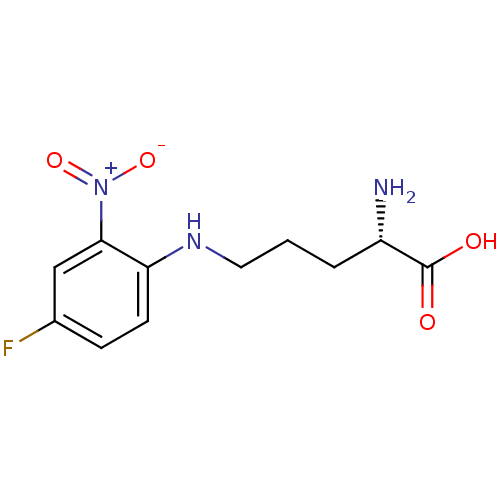 ((S)-2-Amino-5-(4-fluoro-2-nitro-phenylamino)-penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of conversion of radiolabeled arginine to citrulline by isomeric form of nitric oxide synthase Endothelial nitric oxide synthase ... | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065356 ((S)-2-Amino-5-(2-chloro-6-nitro-phenylamino)-penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065358 ((S)-2-Amino-5-(4-fluoro-2-nitro-phenylamino)-penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against isomeric form of Neuronal nitric oxide synthase measured by citrulline assay | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065368 ((S)-2-Amino-5-(2-nitro-4-trifluoromethyl-phenylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against isomeric form of Neuronal nitric oxide synthase measured by citrulline assay | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065357 ((S)-2-Amino-5-m-tolylsulfanyl-pentanoic acid | CHE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065365 ((S)-2-Amino-4-(5-methyl-2-nitro-phenylamino)-butyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065358 ((S)-2-Amino-5-(4-fluoro-2-nitro-phenylamino)-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065360 ((S)-2-Amino-5-(5-hydroxy-2-nitro-phenylamino)-pent...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50065367 ((S)-2-Amino-5-(2-nitro-phenylamino)-pentanoic acid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of conversion of radiolabeled arginine to citrulline by isomeric form of nitric oxide synthase Endothelial nitric oxide synthase ... | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||