

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DADLE from delta receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DAMGO from mu receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]U-69593 from kappa receptor in guinea pig brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50291697 ((1S,2S,13S)-22-(cyclopropylmethyl)-14-oxa-11,22-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DADLE from delta receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50069179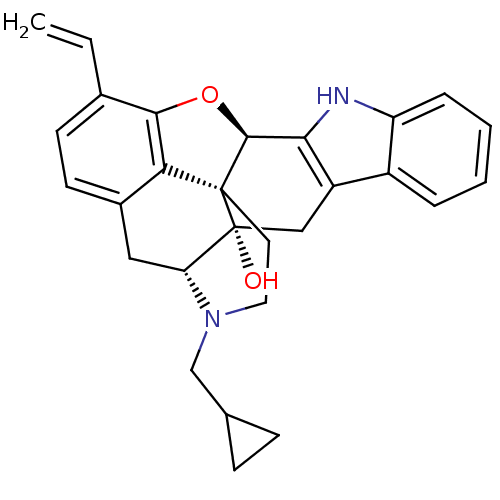 (22-cyclopropylmethyl-16-vinyl-14-oxa-11,22-diazahe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DADLE from delta receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50069175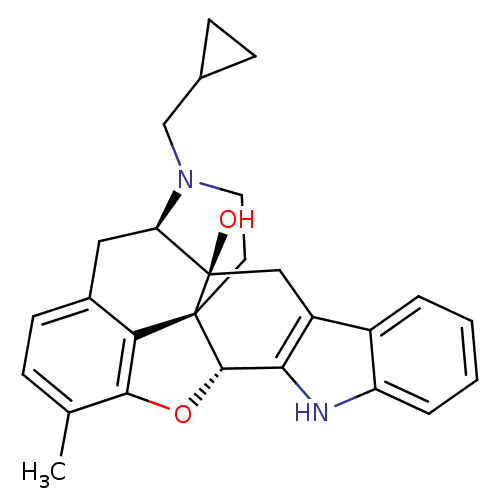 (22-cyclopropylmethyl-16-methyl-14-oxa-11,22-diazah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DADLE from delta receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50069177 (22-cyclopropylmethyl-16-(2-furyl)-14-oxa-11,22-dia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 959 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DADLE from delta receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50069176 (22-cyclopropylmethyl-16-(3-hydroxyphenyl)-14-oxa-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DADLE from delta receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50069177 (22-cyclopropylmethyl-16-(2-furyl)-14-oxa-11,22-dia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DAMGO from mu receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50069181 (22-cyclopropylmethyl-16-phenyl-14-oxa-11,22-diazah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DAMGO from mu receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50291697 ((1S,2S,13S)-22-(cyclopropylmethyl)-14-oxa-11,22-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | >5.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DAMGO from mu receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50069176 (22-cyclopropylmethyl-16-(3-hydroxyphenyl)-14-oxa-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DAMGO from mu receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50069175 (22-cyclopropylmethyl-16-methyl-14-oxa-11,22-diazah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >5.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DAMGO from mu receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50069180 (22-cyclopropylmethyl-16-(3-methoxyphenyl)-14-oxa-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DAMGO from mu receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50069179 (22-cyclopropylmethyl-16-vinyl-14-oxa-11,22-diazahe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DAMGO from mu receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50069180 (22-cyclopropylmethyl-16-(3-methoxyphenyl)-14-oxa-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >6.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DADLE from delta receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50069181 (22-cyclopropylmethyl-16-phenyl-14-oxa-11,22-diazah...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]U-69593 from kappa receptor in guinea pig brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50069177 (22-cyclopropylmethyl-16-(2-furyl)-14-oxa-11,22-dia...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]U-69593 from kappa receptor in guinea pig brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50069176 (22-cyclopropylmethyl-16-(3-hydroxyphenyl)-14-oxa-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]U-69593 from kappa receptor in guinea pig brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50069180 (22-cyclopropylmethyl-16-(3-methoxyphenyl)-14-oxa-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]U-69593 from kappa receptor in guinea pig brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50069175 (22-cyclopropylmethyl-16-methyl-14-oxa-11,22-diazah...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]U-69593 from kappa receptor in guinea pig brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50069181 (22-cyclopropylmethyl-16-phenyl-14-oxa-11,22-diazah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]DADLE from delta receptor in rat brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50291697 ((1S,2S,13S)-22-(cyclopropylmethyl)-14-oxa-11,22-di...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]U-69593 from kappa receptor in guinea pig brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50069179 (22-cyclopropylmethyl-16-vinyl-14-oxa-11,22-diazahe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]U-69593 from kappa receptor in guinea pig brain. | Bioorg Med Chem Lett 8: 799-804 (1999) BindingDB Entry DOI: 10.7270/Q2SF2WPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||