 Found 35 hits of Enzyme Inhibition Constant Data
Found 35 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071702
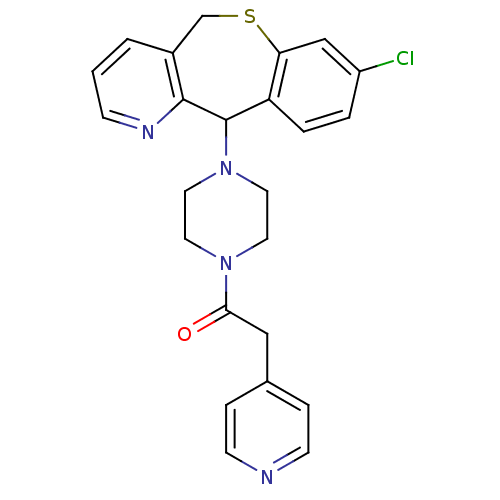
(1-[4-(8-Chloro-5,11-dihydro-10-thia-4-aza-dibenzo[...)Show SMILES Clc1ccc2C(N3CCN(CC3)C(=O)Cc3ccncc3)c3ncccc3CSc2c1 Show InChI InChI=1S/C24H23ClN4OS/c25-19-3-4-20-21(15-19)31-16-18-2-1-7-27-23(18)24(20)29-12-10-28(11-13-29)22(30)14-17-5-8-26-9-6-17/h1-9,15,24H,10-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071702

(1-[4-(8-Chloro-5,11-dihydro-10-thia-4-aza-dibenzo[...)Show SMILES Clc1ccc2C(N3CCN(CC3)C(=O)Cc3ccncc3)c3ncccc3CSc2c1 Show InChI InChI=1S/C24H23ClN4OS/c25-19-3-4-20-21(15-19)31-16-18-2-1-7-27-23(18)24(20)29-12-10-28(11-13-29)22(30)14-17-5-8-26-9-6-17/h1-9,15,24H,10-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50061441

(1-[4-(8-Chloro-6,11-dihydro-5H-benzo[5,6]cyclohept...)Show SMILES Clc1ccc2C(N3CCN(CC3)C(=O)Cc3ccncc3)c3ncccc3CCc2c1 Show InChI InChI=1S/C25H25ClN4O/c26-21-5-6-22-20(17-21)4-3-19-2-1-9-28-24(19)25(22)30-14-12-29(13-15-30)23(31)16-18-7-10-27-11-8-18/h1-2,5-11,17,25H,3-4,12-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071703
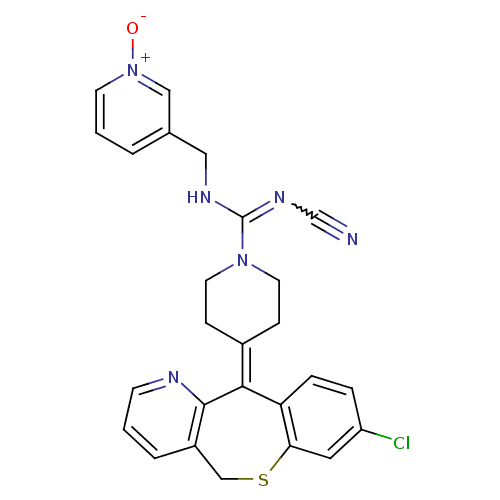
(CHEMBL311434 | Cyanoguanidine derivative)Show SMILES [#8-]-[n+]1cccc(-[#6]-[#7]-[#6](=[#7]C#N)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2/c3ccc(Cl)cc3-[#16]-[#6]-c3cccnc-23)c1 |w:9.9| Show InChI InChI=1S/C26H23ClN6OS/c27-21-5-6-22-23(13-21)35-16-20-4-1-9-29-25(20)24(22)19-7-11-32(12-8-19)26(31-17-28)30-14-18-3-2-10-33(34)15-18/h1-6,9-10,13,15H,7-8,11-12,14,16H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071700

(CHEMBL81889 | Cyanoguanidine derivative)Show SMILES Clc1ccc2c(-[#16]-[#6]-c3cccnc3\[#6]-2=[#6]-2\[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](-[#7]-[#6]-c2ccncc2)=[#7]C#N)c1 |w:30.34| Show InChI InChI=1S/C26H23ClN6S/c27-21-3-4-22-23(14-21)34-16-20-2-1-9-30-25(20)24(22)19-7-12-33(13-8-19)26(32-17-28)31-15-18-5-10-29-11-6-18/h1-6,9-11,14H,7-8,12-13,15-16H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071699
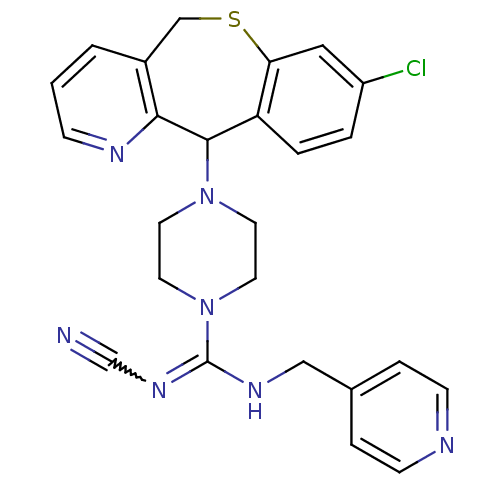
(CHEMBL313090 | Cyanoguanidine derivative)Show SMILES Clc1ccc2C(N3CCN(CC3)C(NCc3ccncc3)=NC#N)c3ncccc3CSc2c1 |w:21.23| Show InChI InChI=1S/C25H24ClN7S/c26-20-3-4-21-22(14-20)34-16-19-2-1-7-29-23(19)24(21)32-10-12-33(13-11-32)25(31-17-27)30-15-18-5-8-28-9-6-18/h1-9,14,24H,10-13,15-16H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071710
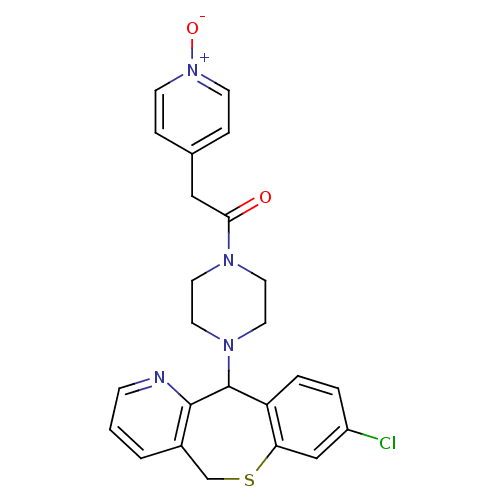
(1-[4-(8-Chloro-5,11-dihydro-10-thia-4-aza-dibenzo[...)Show SMILES [O-][n+]1ccc(CC(=O)N2CCN(CC2)C2c3ccc(Cl)cc3SCc3cccnc23)cc1 Show InChI InChI=1S/C24H23ClN4O2S/c25-19-3-4-20-21(15-19)32-16-18-2-1-7-26-23(18)24(20)28-12-10-27(11-13-28)22(30)14-17-5-8-29(31)9-6-17/h1-9,15,24H,10-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071712
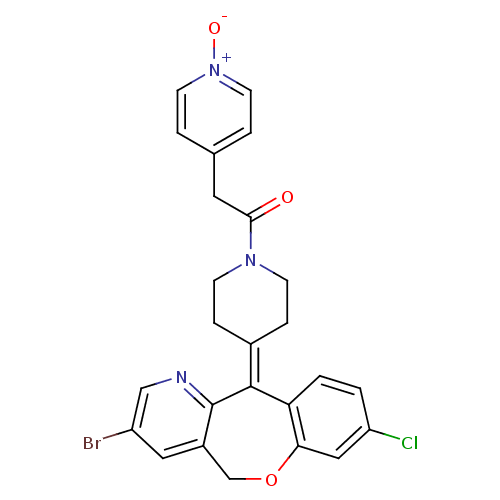
(1-[4-(2-Bromo-8-chloro-11H-10-oxa-4-aza-dibenzo[a,...)Show SMILES [#8-]-[n+]1ccc(-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2\c3ccc(Cl)cc3-[#8]-[#6]-c3cc(Br)cnc-23)cc1 Show InChI InChI=1S/C25H21BrClN3O3/c26-19-12-18-15-33-22-13-20(27)1-2-21(22)24(25(18)28-14-19)17-5-7-29(8-6-17)23(31)11-16-3-9-30(32)10-4-16/h1-4,9-10,12-14H,5-8,11,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071718
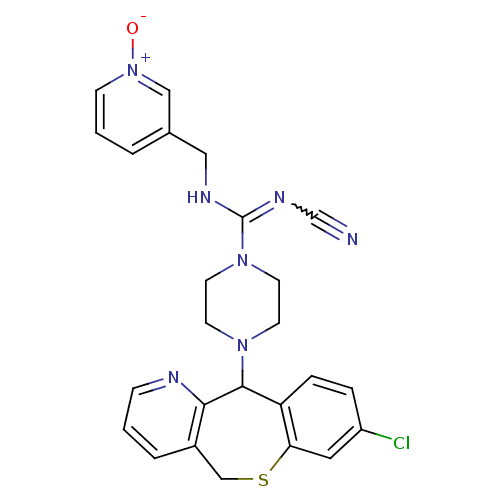
(CHEMBL421722 | Cyanoguanidine derivative)Show SMILES [O-][n+]1cccc(CNC(=NC#N)N2CCN(CC2)C2c3ccc(Cl)cc3SCc3cccnc23)c1 |w:9.9| Show InChI InChI=1S/C25H24ClN7OS/c26-20-5-6-21-22(13-20)35-16-19-4-1-7-28-23(19)24(21)31-9-11-32(12-10-31)25(30-17-27)29-14-18-3-2-8-33(34)15-18/h1-8,13,15,24H,9-12,14,16H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071709
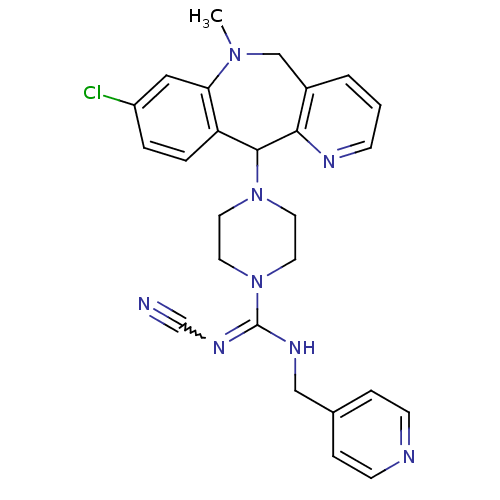
(CHEMBL87345 | Cyanoguanidine derivative)Show SMILES CN1Cc2cccnc2C(N2CCN(CC2)C(NCc2ccncc2)=NC#N)c2ccc(Cl)cc12 |w:25.28| Show InChI InChI=1S/C26H27ClN8/c1-33-17-20-3-2-8-30-24(20)25(22-5-4-21(27)15-23(22)33)34-11-13-35(14-12-34)26(32-18-28)31-16-19-6-9-29-10-7-19/h2-10,15,25H,11-14,16-17H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071715
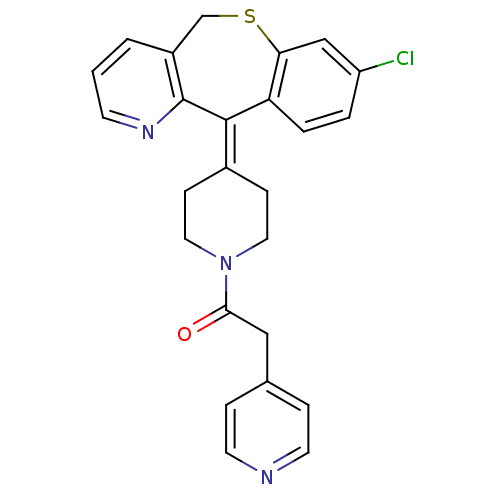
(1-[4-(8-Chloro-11H-10-thia-4-aza-dibenzo[a,d]cyclo...)Show SMILES Clc1ccc2c(-[#16]-[#6]-c3cccnc3\[#6]-2=[#6]-2/[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-c2ccncc2)c1 Show InChI InChI=1S/C25H22ClN3OS/c26-20-3-4-21-22(15-20)31-16-19-2-1-9-28-25(19)24(21)18-7-12-29(13-8-18)23(30)14-17-5-10-27-11-6-17/h1-6,9-11,15H,7-8,12-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14454

(1-(4-pyridylacetyl)-4-(8-chloro-5,6-dihydro-11H-be...)Show SMILES Clc1ccc2c(-[#6]-[#6]-c3cccnc3\[#6]-2=[#6]-2\[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-c2ccncc2)c1 Show InChI InChI=1S/C26H24ClN3O/c27-22-5-6-23-21(17-22)4-3-20-2-1-11-29-26(20)25(23)19-9-14-30(15-10-19)24(31)16-18-7-12-28-13-8-18/h1-2,5-8,11-13,17H,3-4,9-10,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071698
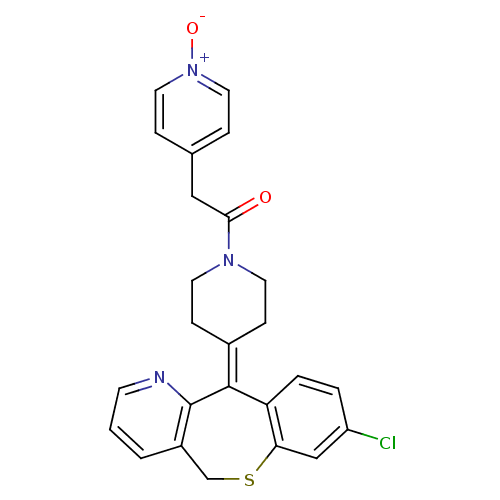
(1-[4-(8-Chloro-11H-10-thia-4-aza-dibenzo[a,d]cyclo...)Show SMILES [#8-]-[n+]1ccc(-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2\c3ccc(Cl)cc3-[#16]-[#6]-c3cccnc-23)cc1 Show InChI InChI=1S/C25H22ClN3O2S/c26-20-3-4-21-22(15-20)32-16-19-2-1-9-27-25(19)24(21)18-7-10-28(11-8-18)23(30)14-17-5-12-29(31)13-6-17/h1-6,9,12-13,15H,7-8,10-11,14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071705
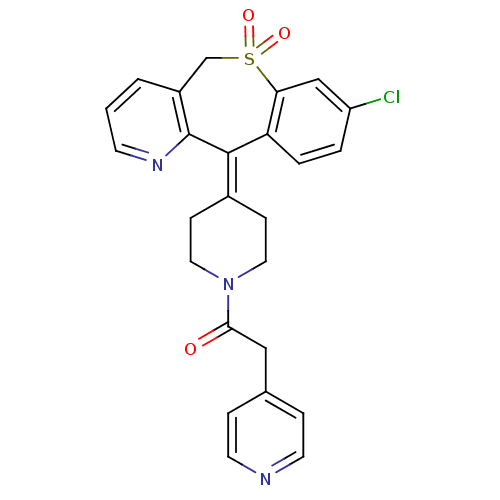
(1-[4-(8-Chloro-10,10-dioxo-10,11-dihydro-10lambda*...)Show SMILES Clc1ccc2c(c1)S(=O)(=O)[#6]-c1cccnc1\[#6]-2=[#6]-1/[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#6]-c1ccncc1 Show InChI InChI=1S/C25H22ClN3O3S/c26-20-3-4-21-22(15-20)33(31,32)16-19-2-1-9-28-25(19)24(21)18-7-12-29(13-8-18)23(30)14-17-5-10-27-11-6-17/h1-6,9-11,15H,7-8,12-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071716
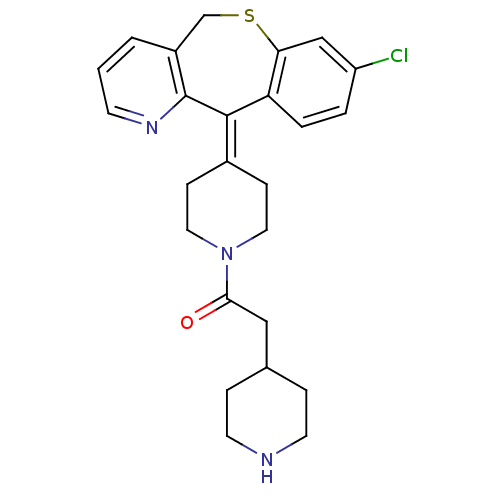
(1-[4-(8-Chloro-11H-10-thia-4-aza-dibenzo[a,d]cyclo...)Show SMILES Clc1ccc2c(-[#16]-[#6]-c3cccnc3\[#6]-2=[#6]-2/[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-[#6]-2-[#6]-[#6]-[#7]-[#6]-[#6]-2)c1 Show InChI InChI=1S/C25H28ClN3OS/c26-20-3-4-21-22(15-20)31-16-19-2-1-9-28-25(19)24(21)18-7-12-29(13-8-18)23(30)14-17-5-10-27-11-6-17/h1-4,9,15,17,27H,5-8,10-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071711
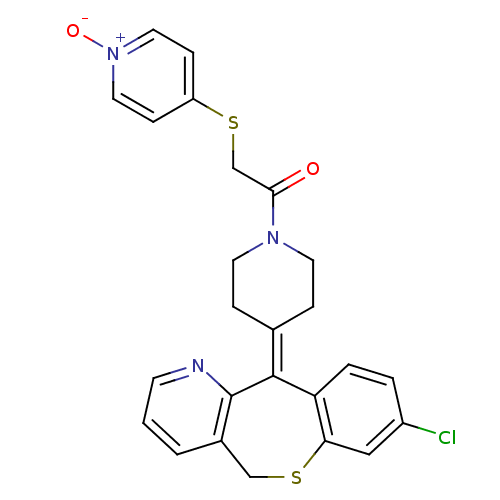
(1-[4-(8-Chloro-11H-10-thia-4-aza-dibenzo[a,d]cyclo...)Show SMILES [#8-]-[n+]1ccc(-[#16]-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2/c3ccc(Cl)cc3-[#16]-[#6]-c3cccnc-23)cc1 Show InChI InChI=1S/C25H22ClN3O2S2/c26-19-3-4-21-22(14-19)33-15-18-2-1-9-27-25(18)24(21)17-5-10-28(11-6-17)23(30)16-32-20-7-12-29(31)13-8-20/h1-4,7-9,12-14H,5-6,10-11,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071714
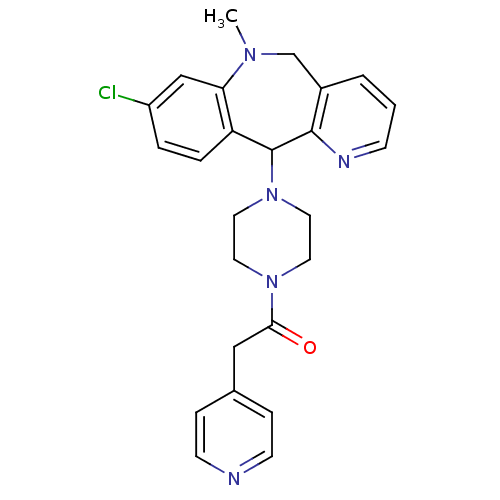
(1-[4-(8-Chloro-10-methyl-10,11-dihydro-5H-4,10-dia...)Show SMILES CN1Cc2cccnc2C(N2CCN(CC2)C(=O)Cc2ccncc2)c2ccc(Cl)cc12 Show InChI InChI=1S/C25H26ClN5O/c1-29-17-19-3-2-8-28-24(19)25(21-5-4-20(26)16-22(21)29)31-13-11-30(12-14-31)23(32)15-18-6-9-27-10-7-18/h2-10,16,25H,11-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071708

(4-{2-[4-(8-Chloro-11H-10-thia-4-aza-dibenzo[a,d]cy...)Show SMILES [#7]-[#6](=O)-[#7]-1-[#6]-[#6]-[#6](-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2\c3ccc(Cl)cc3-[#16]-[#6]-c3cccnc-23)-[#6]-[#6]-1 Show InChI InChI=1S/C26H29ClN4O2S/c27-20-3-4-21-22(15-20)34-16-19-2-1-9-29-25(19)24(21)18-7-12-30(13-8-18)23(32)14-17-5-10-31(11-6-17)26(28)33/h1-4,9,15,17H,5-8,10-14,16H2,(H2,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071720
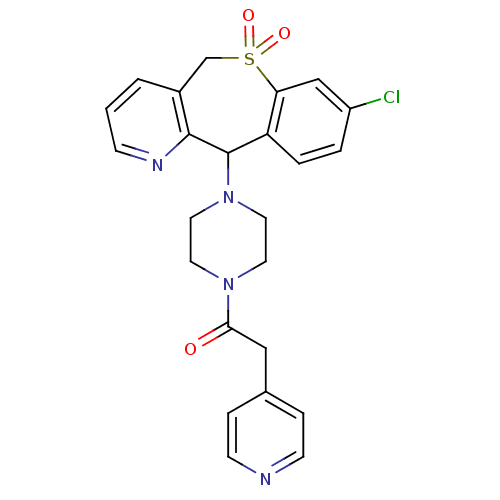
(1-[4-(8-Chloro-10,10-dioxo-10,11-dihydro-5H-10lamb...)Show SMILES Clc1ccc2C(N3CCN(CC3)C(=O)Cc3ccncc3)c3ncccc3CS(=O)(=O)c2c1 Show InChI InChI=1S/C24H23ClN4O3S/c25-19-3-4-20-21(15-19)33(31,32)16-18-2-1-7-27-23(18)24(20)29-12-10-28(11-13-29)22(30)14-17-5-8-26-9-6-17/h1-9,15,24H,10-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM14454

(1-(4-pyridylacetyl)-4-(8-chloro-5,6-dihydro-11H-be...)Show SMILES Clc1ccc2c(-[#6]-[#6]-c3cccnc3\[#6]-2=[#6]-2\[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-c2ccncc2)c1 Show InChI InChI=1S/C26H24ClN3O/c27-22-5-6-23-21(17-22)4-3-20-2-1-11-29-26(20)25(23)19-9-14-30(15-10-19)24(31)16-18-7-12-28-13-8-18/h1-2,5-8,11-13,17H,3-4,9-10,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of Ras processing in COS cells |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071701
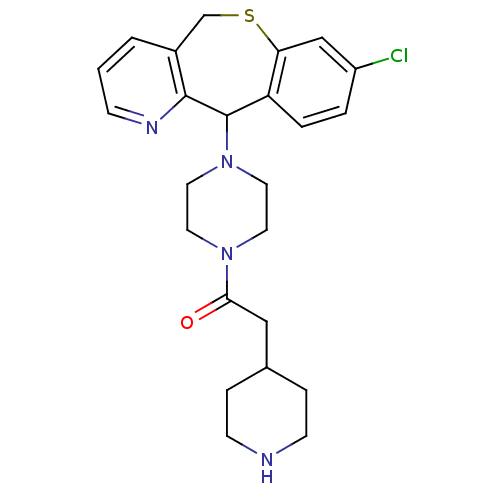
(1-[4-(8-Chloro-5,11-dihydro-10-thia-4-aza-dibenzo[...)Show SMILES Clc1ccc2C(N3CCN(CC3)C(=O)CC3CCNCC3)c3ncccc3CSc2c1 Show InChI InChI=1S/C24H29ClN4OS/c25-19-3-4-20-21(15-19)31-16-18-2-1-7-27-23(18)24(20)29-12-10-28(11-13-29)22(30)14-17-5-8-26-9-6-17/h1-4,7,15,17,24,26H,5-6,8-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071704
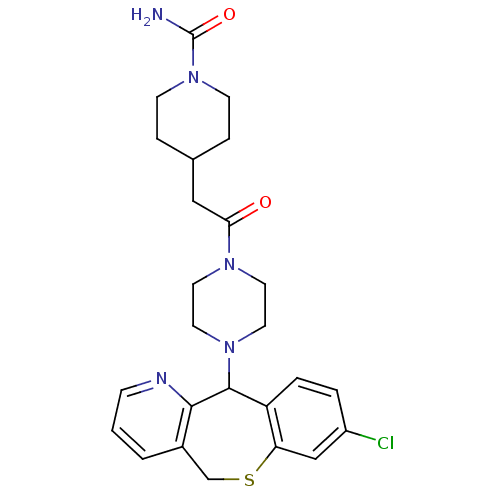
(4-{2-[4-(8-Chloro-5,11-dihydro-10-thia-4-aza-diben...)Show SMILES NC(=O)N1CCC(CC(=O)N2CCN(CC2)C2c3ccc(Cl)cc3SCc3cccnc23)CC1 Show InChI InChI=1S/C25H30ClN5O2S/c26-19-3-4-20-21(15-19)34-16-18-2-1-7-28-23(18)24(20)30-12-10-29(11-13-30)22(32)14-17-5-8-31(9-6-17)25(27)33/h1-4,7,15,17,24H,5-6,8-14,16H2,(H2,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071717

(1-[4-(8-Chloro-11H-10-oxa-4-aza-dibenzo[a,d]cycloh...)Show SMILES [#8-]-[n+]1ccc(-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2\c3ccc(Cl)cc3-[#8]-[#6]-c3cccnc-23)cc1 Show InChI InChI=1S/C25H22ClN3O3/c26-20-3-4-21-22(15-20)32-16-19-2-1-9-27-25(19)24(21)18-7-10-28(11-8-18)23(30)14-17-5-12-29(31)13-6-17/h1-6,9,12-13,15H,7-8,10-11,14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071700

(CHEMBL81889 | Cyanoguanidine derivative)Show SMILES Clc1ccc2c(-[#16]-[#6]-c3cccnc3\[#6]-2=[#6]-2\[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](-[#7]-[#6]-c2ccncc2)=[#7]C#N)c1 |w:30.34| Show InChI InChI=1S/C26H23ClN6S/c27-21-3-4-22-23(14-21)34-16-20-2-1-9-30-25(20)24(22)19-7-12-33(13-8-19)26(32-17-28)31-15-18-5-10-29-11-6-18/h1-6,9-11,14H,7-8,12-13,15-16H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of Ras processing in COS cells |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071706

(1-[4-(8-Chloro-11H-10-thia-4-aza-dibenzo[a,d]cyclo...)Show SMILES [#6]-[#7]-1-[#6]-[#6]-[#6](-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2/c3ccc(Cl)cc3-[#16]-[#6]-c3cccnc-23)-[#6]-[#6]-1 Show InChI InChI=1S/C26H30ClN3OS/c1-29-11-6-18(7-12-29)15-24(31)30-13-8-19(9-14-30)25-22-5-4-21(27)16-23(22)32-17-20-3-2-10-28-26(20)25/h2-5,10,16,18H,6-9,11-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071707

(1-[4-(8-Chloro-5,11-dihydro-10-thia-4-aza-dibenzo[...)Show SMILES CN1CCC(CC(=O)N2CCN(CC2)C2c3ccc(Cl)cc3SCc3cccnc23)CC1 Show InChI InChI=1S/C25H31ClN4OS/c1-28-9-6-18(7-10-28)15-23(31)29-11-13-30(14-12-29)25-21-5-4-20(26)16-22(21)32-17-19-3-2-8-27-24(19)25/h2-5,8,16,18,25H,6-7,9-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071713
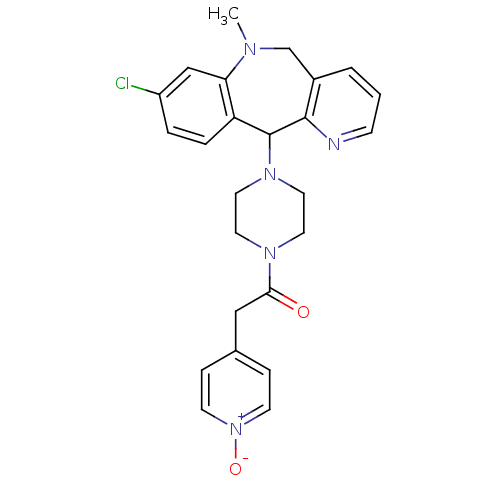
(1-[4-(8-Chloro-10-methyl-10,11-dihydro-5H-4,10-dia...)Show SMILES CN1Cc2cccnc2C(N2CCN(CC2)C(=O)Cc2cc[n+]([O-])cc2)c2ccc(Cl)cc12 Show InChI InChI=1S/C25H26ClN5O2/c1-28-17-19-3-2-8-27-24(19)25(21-5-4-20(26)16-22(21)28)30-13-11-29(12-14-30)23(32)15-18-6-9-31(33)10-7-18/h2-10,16,25H,11-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071719

(1-[4-(8-Chloro-10,10-dioxo-10,11-dihydro-5H-10lamb...)Show SMILES [O-][n+]1ccc(CC(=O)N2CCN(CC2)C2c3ccc(Cl)cc3S(=O)(=O)Cc3cccnc23)cc1 Show InChI InChI=1S/C24H23ClN4O4S/c25-19-3-4-20-21(15-19)34(32,33)16-18-2-1-7-26-23(18)24(20)28-12-10-27(11-13-28)22(30)14-17-5-8-29(31)9-6-17/h1-9,15,24H,10-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro FPT potency by measuring the ability to inhibit the transfer of [3H]-farnesyl from farnesyl pyrophosphate to H-Ras-CLVS. |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50061441

(1-[4-(8-Chloro-6,11-dihydro-5H-benzo[5,6]cyclohept...)Show SMILES Clc1ccc2C(N3CCN(CC3)C(=O)Cc3ccncc3)c3ncccc3CCc2c1 Show InChI InChI=1S/C25H25ClN4O/c26-21-5-6-22-20(17-21)4-3-19-2-1-9-28-24(19)25(22)30-14-12-29(13-15-30)23(31)16-18-7-10-27-11-8-18/h1-2,5-11,17,25H,3-4,12-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of Ras processing in COS cells |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071708

(4-{2-[4-(8-Chloro-11H-10-thia-4-aza-dibenzo[a,d]cy...)Show SMILES [#7]-[#6](=O)-[#7]-1-[#6]-[#6]-[#6](-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2\c3ccc(Cl)cc3-[#16]-[#6]-c3cccnc-23)-[#6]-[#6]-1 Show InChI InChI=1S/C26H29ClN4O2S/c27-20-3-4-21-22(15-20)34-16-19-2-1-9-29-25(19)24(21)18-7-12-30(13-8-18)23(32)14-17-5-10-31(11-6-17)26(28)33/h1-4,9,15,17H,5-8,10-14,16H2,(H2,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of Ras processing in COS cells |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071715

(1-[4-(8-Chloro-11H-10-thia-4-aza-dibenzo[a,d]cyclo...)Show SMILES Clc1ccc2c(-[#16]-[#6]-c3cccnc3\[#6]-2=[#6]-2/[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-c2ccncc2)c1 Show InChI InChI=1S/C25H22ClN3OS/c26-20-3-4-21-22(15-20)31-16-19-2-1-9-28-25(19)24(21)18-7-12-29(13-8-18)23(30)14-17-5-10-27-11-6-17/h1-6,9-11,15H,7-8,12-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of Ras processing in COS cells |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071711

(1-[4-(8-Chloro-11H-10-thia-4-aza-dibenzo[a,d]cyclo...)Show SMILES [#8-]-[n+]1ccc(-[#16]-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2/c3ccc(Cl)cc3-[#16]-[#6]-c3cccnc-23)cc1 Show InChI InChI=1S/C25H22ClN3O2S2/c26-19-3-4-21-22(14-19)33-15-18-2-1-9-27-25(18)24(21)17-5-10-28(11-6-17)23(30)16-32-20-7-12-29(31)13-8-20/h1-4,7-9,12-14H,5-6,10-11,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of Ras processing in COS cells |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071710

(1-[4-(8-Chloro-5,11-dihydro-10-thia-4-aza-dibenzo[...)Show SMILES [O-][n+]1ccc(CC(=O)N2CCN(CC2)C2c3ccc(Cl)cc3SCc3cccnc23)cc1 Show InChI InChI=1S/C24H23ClN4O2S/c25-19-3-4-20-21(15-19)32-16-18-2-1-7-26-23(18)24(20)28-12-10-27(11-13-28)22(30)14-17-5-8-29(31)9-6-17/h1-9,15,24H,10-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of Ras processing in COS cells |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071698

(1-[4-(8-Chloro-11H-10-thia-4-aza-dibenzo[a,d]cyclo...)Show SMILES [#8-]-[n+]1ccc(-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]-2\c3ccc(Cl)cc3-[#16]-[#6]-c3cccnc-23)cc1 Show InChI InChI=1S/C25H22ClN3O2S/c26-20-3-4-21-22(15-20)32-16-19-2-1-9-27-25(19)24(21)18-7-10-28(11-8-18)23(30)14-17-5-12-29(31)13-6-17/h1-6,9,12-13,15H,7-8,10-11,14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of Ras processing in COS cells |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50071713

(1-[4-(8-Chloro-10-methyl-10,11-dihydro-5H-4,10-dia...)Show SMILES CN1Cc2cccnc2C(N2CCN(CC2)C(=O)Cc2cc[n+]([O-])cc2)c2ccc(Cl)cc12 Show InChI InChI=1S/C25H26ClN5O2/c1-28-17-19-3-2-8-27-24(19)25(21-5-4-20(26)16-22(21)28)30-13-11-29(12-14-30)23(32)15-18-6-9-31(33)10-7-18/h2-10,16,25H,11-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of Ras processing in COS cells |
Bioorg Med Chem Lett 8: 2521-6 (1999)
BindingDB Entry DOI: 10.7270/Q24M93PP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data