

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071982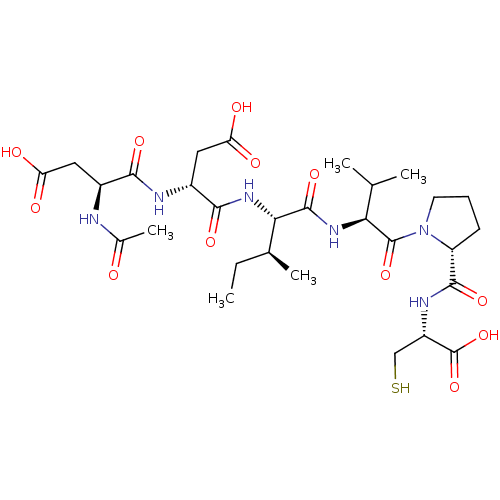 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50071966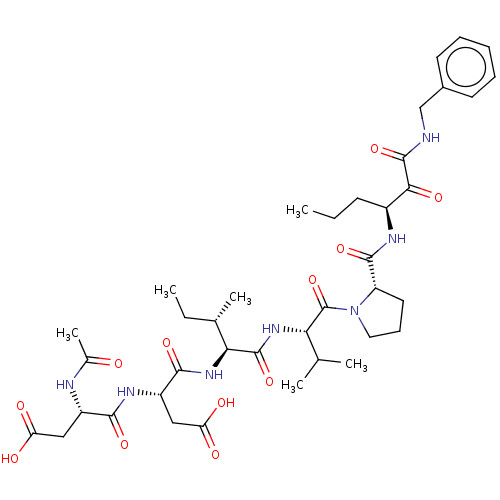 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human liver Cathepsin B | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50071966 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human leucocyte elastase (HLE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50071966 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of porcine pancreatic elastase (PPE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50071973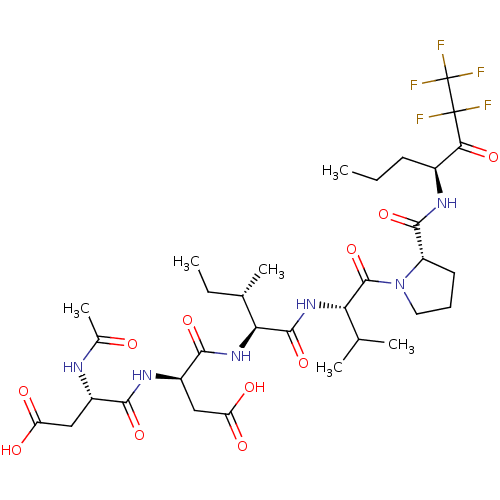 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of porcine pancreatic elastase (PPE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50071968 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of porcine pancreatic elastase (PPE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50071973 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human leucocyte elastase (HLE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50071983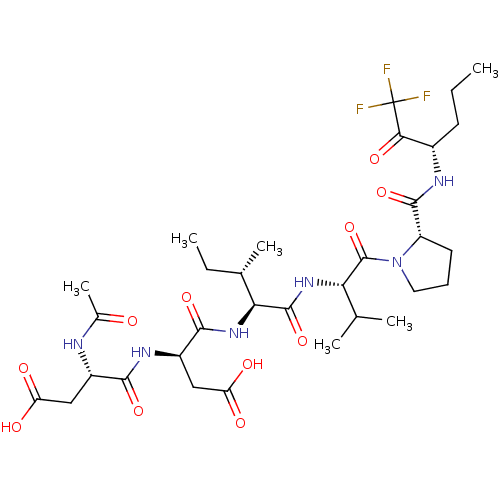 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human leucocyte elastase (HLE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50071983 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of porcine pancreatic elastase (PPE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071968 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071965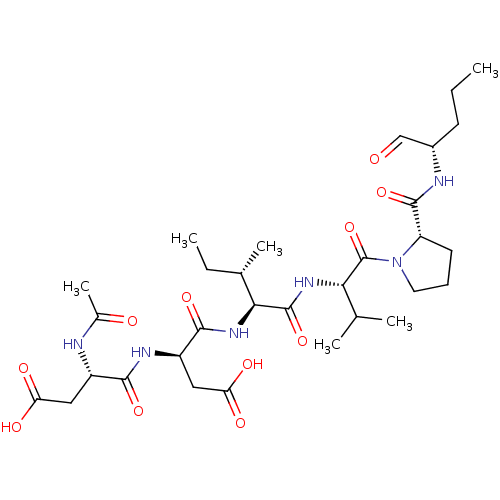 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071966 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50071965 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of porcine pancreatic elastase (PPE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50071965 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human leucocyte elastase (HLE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071970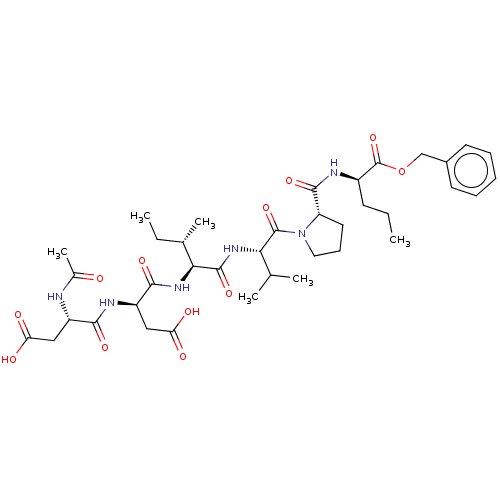 ((R)-2-{[1-((S)-2-{(2S,3S)-2-[(R)-2-((S)-2-Acetylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50071965 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human liver Cathepsin B | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071978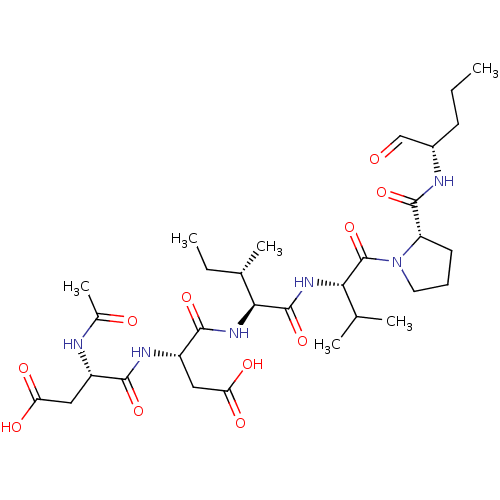 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for competitive inhibition of hepatitis C virus (HCV) serine protease | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071973 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071975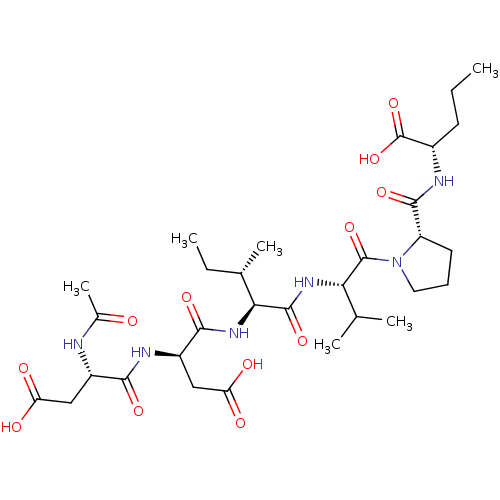 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071964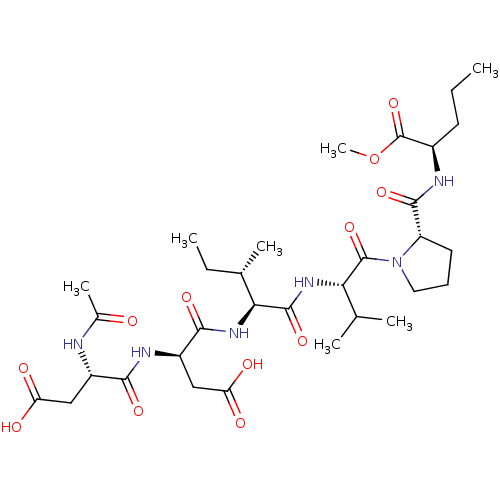 ((R)-2-{[1-((S)-2-{(2S,3S)-2-[(R)-2-((S)-2-Acetylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071983 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071974 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071967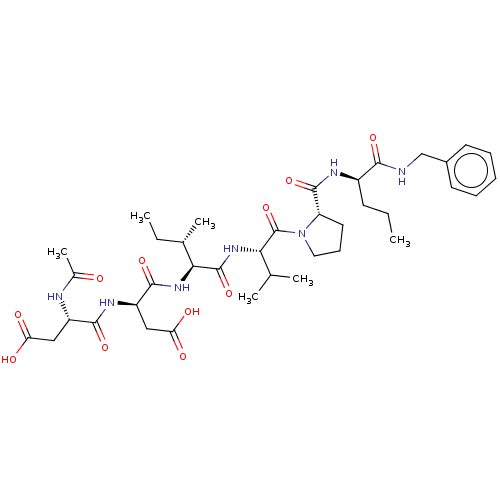 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071963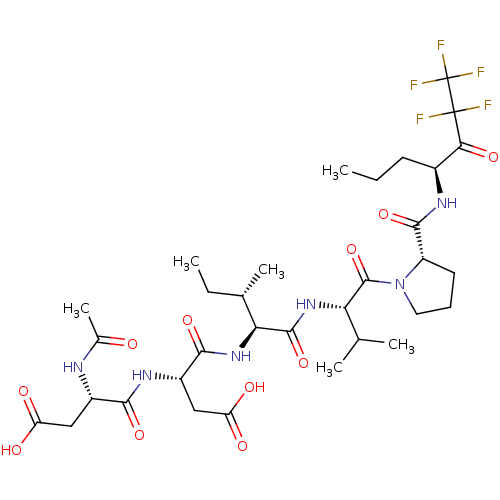 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50071967 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human leucocyte elastase (HLE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071984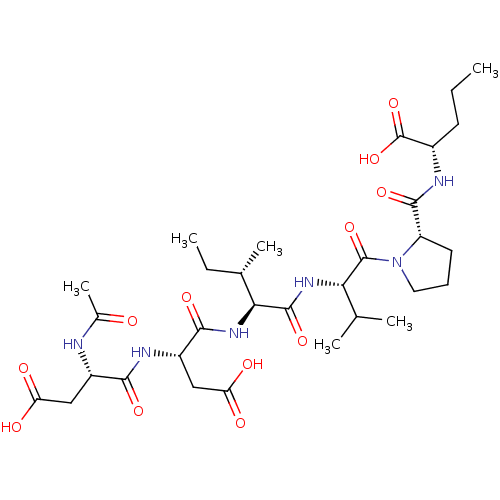 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071962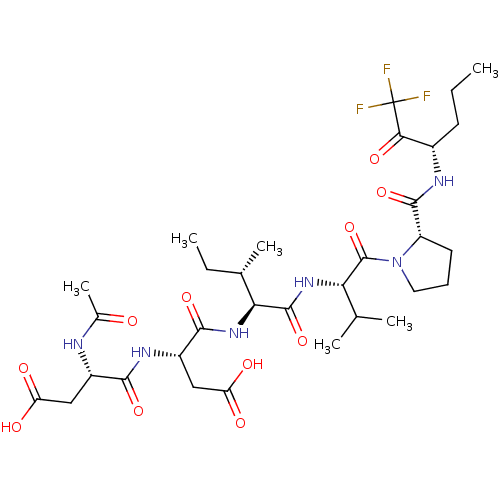 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071971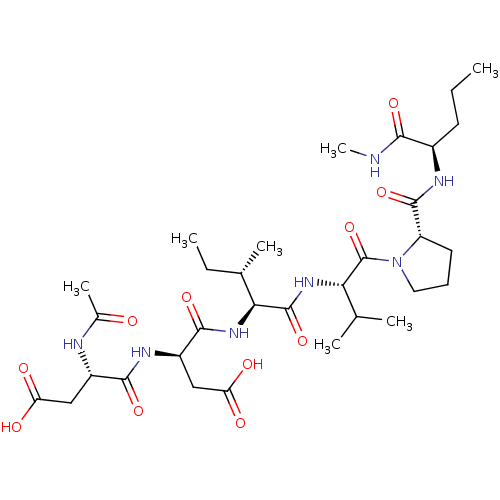 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50071968 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.72E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human leucocyte elastase (HLE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50071968 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.72E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human liver Cathepsin B | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071969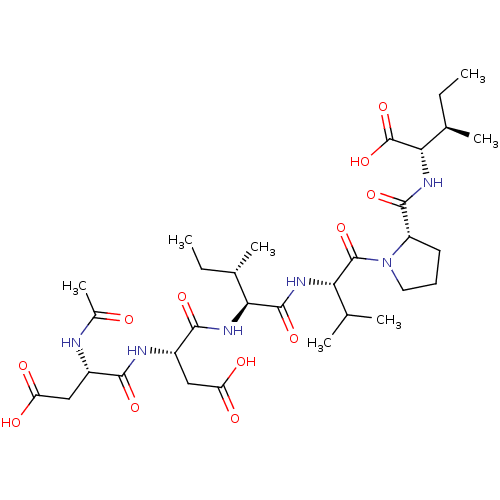 ((2S,3R)-2-{[1-((S)-2-{(2S,3S)-2-[(S)-2-((S)-2-Acet...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50071967 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of porcine pancreatic elastase (PPE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071977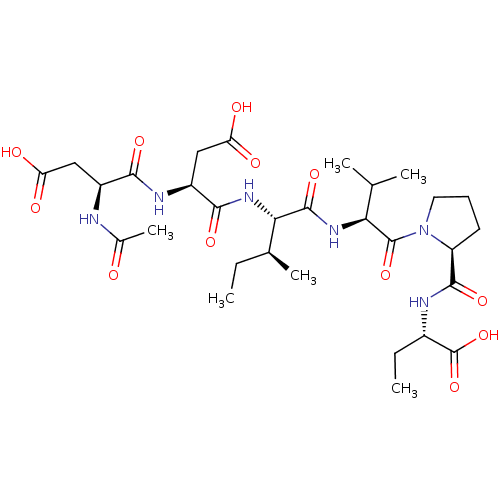 ((S)-2-{[1-((S)-2-{(2S,3S)-2-[(S)-2-((S)-2-Acetylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071976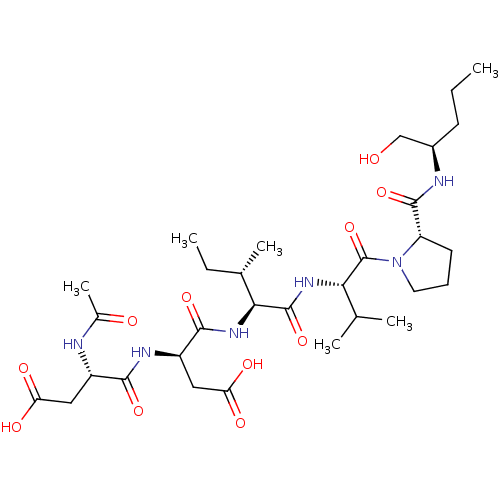 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50071973 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human liver Cathepsin B | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071972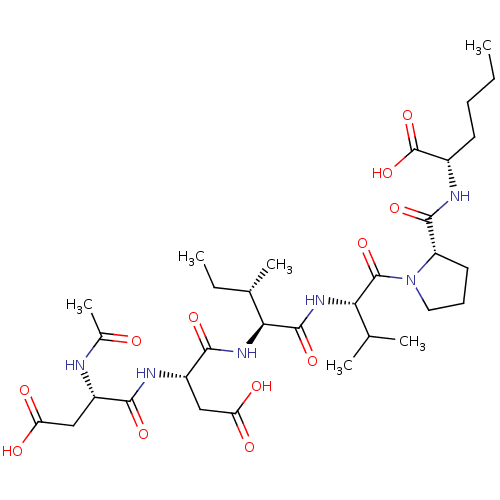 ((S)-2-{[1-((S)-2-{(2S,3S)-2-[(S)-2-((S)-2-Acetylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071961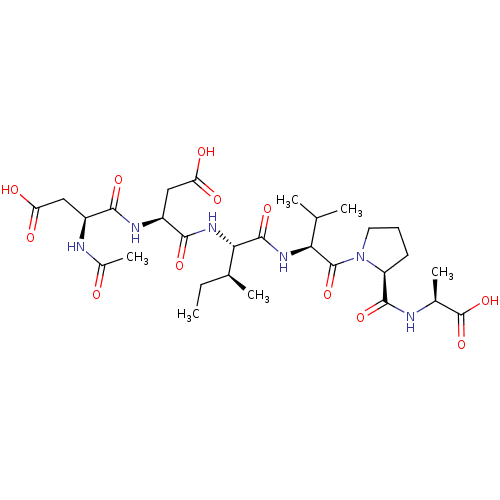 ((S)-2-{[1-((S)-2-{(2S,3S)-2-[(R)-2-((S)-2-Acetylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071981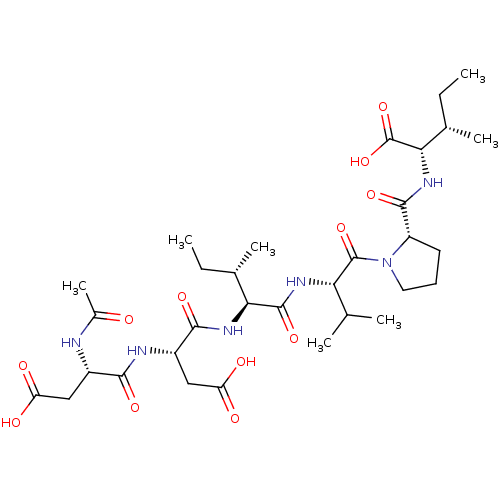 ((2S,3S)-2-{[1-((S)-2-{(2S,3S)-2-[(S)-2-((S)-2-Acet...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071979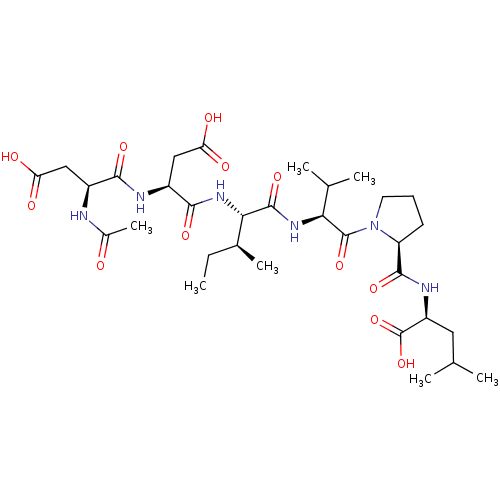 ((S)-2-{[1-((S)-2-{(2S,3S)-2-[(S)-2-((S)-2-Acetylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50071983 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human liver Cathepsin B | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50071975 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human liver Cathepsin B | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50071975 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of porcine pancreatic elastase (PPE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50071975 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human leucocyte elastase (HLE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50071967 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human liver Cathepsin B | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50071970 ((R)-2-{[1-((S)-2-{(2S,3S)-2-[(R)-2-((S)-2-Acetylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human liver Cathepsin B | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50071970 ((R)-2-{[1-((S)-2-{(2S,3S)-2-[(R)-2-((S)-2-Acetylam...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of porcine pancreatic elastase (PPE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50071970 ((R)-2-{[1-((S)-2-{(2S,3S)-2-[(R)-2-((S)-2-Acetylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human leucocyte elastase (HLE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071980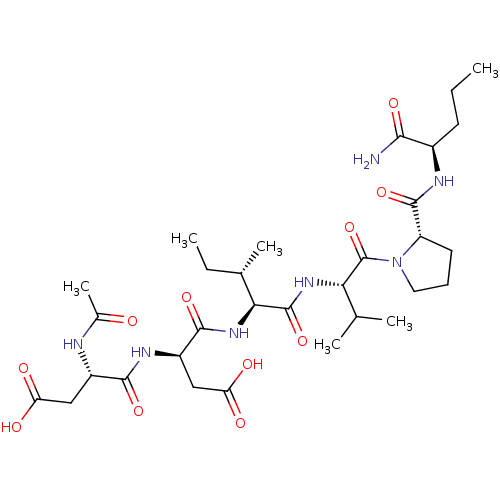 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||