 Found 74 hits of Enzyme Inhibition Constant Data
Found 74 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070692
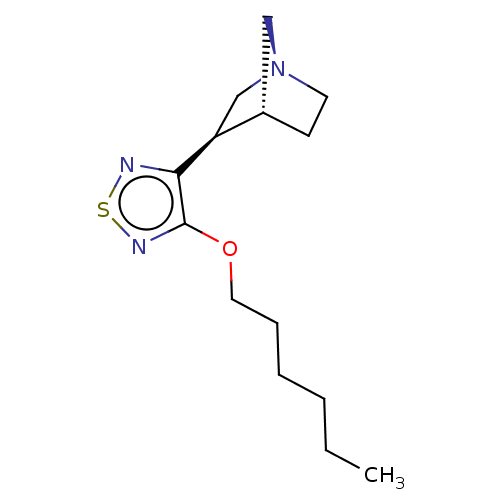
(CHEMBL99240)Show SMILES [H][C@@]12CC[N@@](C[C@@H]1c1nsnc1OCCCCCC)C2 Show InChI InChI=1S/C34H50N8O8/c1-3-20(2)28(42-30(46)24(12-7-8-16-35)39-29(45)23-19-22(43)14-15-27(23)44)32(48)41-26(18-21-10-5-4-6-11-21)31(47)40-25(33(49)50)13-9-17-38-34(36)37/h4-6,10-11,14-15,19-20,24-26,28,43-44H,3,7-9,12-13,16-18,35H2,1-2H3,(H,39,45)(H,40,47)(H,41,48)(H,42,46)(H,49,50)(H4,36,37,38)/t20?,24-,25-,26-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072214
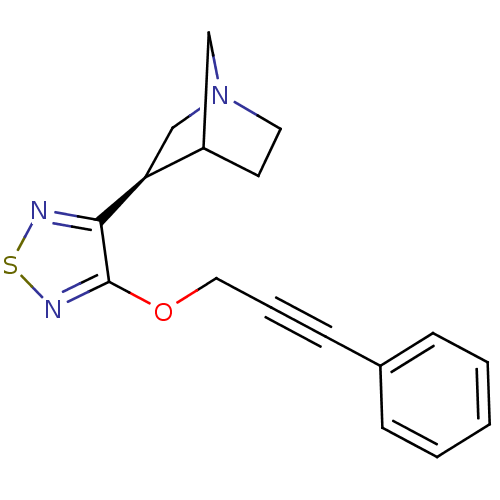
((R)-3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiaz...)Show InChI InChI=1S/C17H17N3OS/c1-2-5-13(6-3-1)7-4-10-21-17-16(18-22-19-17)15-12-20-9-8-14(15)11-20/h1-3,5-6,14-15H,8-12H2/t14?,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070735
(CHEMBL317324)Show InChI InChI=1S/C29H48N8O8/c1-4-16(3)23(27(43)34-19(5-2)28(44)45)37-26(42)21(10-8-14-33-29(31)32)36-25(41)20(9-6-7-13-30)35-24(40)18-15-17(38)11-12-22(18)39/h11-12,15-16,19-21,23,38-39H,4-10,13-14,30H2,1-3H3,(H,34,43)(H,35,40)(H,36,41)(H,37,42)(H,44,45)(H4,31,32,33)/t16?,19?,20-,21-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072227
((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...)Show SMILES C(Oc1nsnc1[C@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3OS/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070725
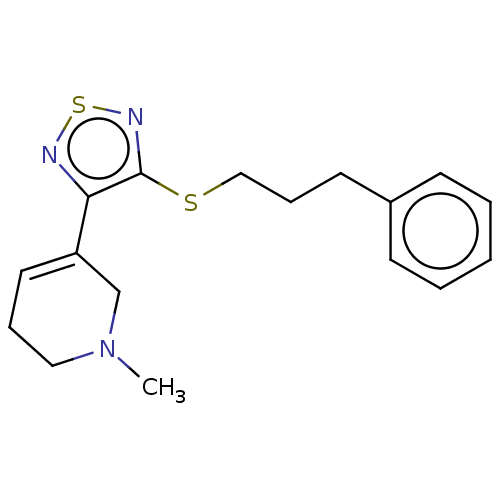
(CHEMBL318403)Show InChI InChI=1S/C28H43N5O8/c1-4-16(3)23(26(38)30-19(5-2)28(40)41)32-25(37)21-10-8-14-33(21)27(39)20(9-6-7-13-29)31-24(36)18-15-17(34)11-12-22(18)35/h11-12,15-16,19-21,23,34-35H,4-10,13-14,29H2,1-3H3,(H,30,38)(H,31,36)(H,32,37)(H,40,41)/t16?,19?,20-,21-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070647
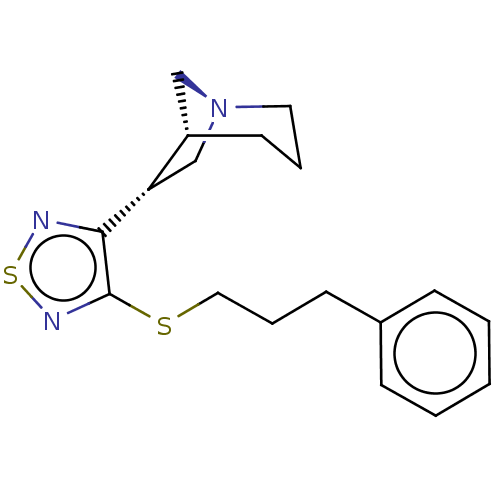
(CHEMBL329924)Show SMILES [H][C@]12C[N@](C[C@@H]1c1nsnc1SCCCc1ccccc1)CCC2 Show InChI InChI=1S/C18H23N5O/c1-3-6-13-7-4-5-8-14(13)9-15(12(2)24)23-11-22-16-17(19)20-10-21-18(16)23/h4-5,7-8,10-12,15,24H,3,6,9H2,1-2H3,(H2,19,20,21)/t12-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003351
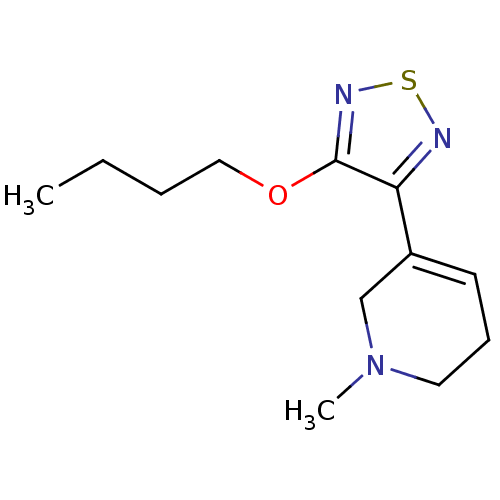
(3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...)Show InChI InChI=1S/C12H19N3OS/c1-3-4-8-16-12-11(13-17-14-12)10-6-5-7-15(2)9-10/h6H,3-5,7-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003369
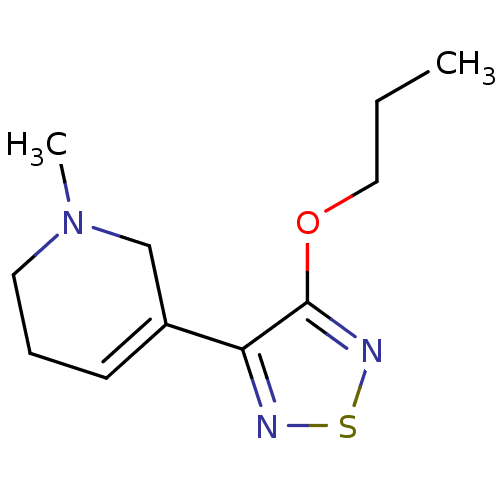
(1-Methyl-5-(4-propoxy-[1,2,5]thiadiazol-3-yl)-1,2,...)Show InChI InChI=1S/C11H17N3OS/c1-3-7-15-11-10(12-16-13-11)9-5-4-6-14(2)8-9/h5H,3-4,6-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070684

(CHEMBL321073)Show SMILES [H][C@@]12CC[N@@](C[C@@H]1c1nsnc1SCCCc1ccccc1)C2 Show InChI InChI=1S/C28H43N7O8/c1-4-15(3)22(25(40)32-18(5-2)27(42)43)34-24(39)20-9-7-13-35(20)26(41)19(8-6-12-31-28(29)30)33-23(38)17-14-16(36)10-11-21(17)37/h10-11,14-15,18-20,22,36-37H,4-9,12-13H2,1-3H3,(H,32,40)(H,33,38)(H,34,39)(H,42,43)(H4,29,30,31)/t15?,18?,19-,20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072225

((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynylsulfanyl)-[1,...)Show SMILES C(Sc1nsnc1[C@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3S2/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003366
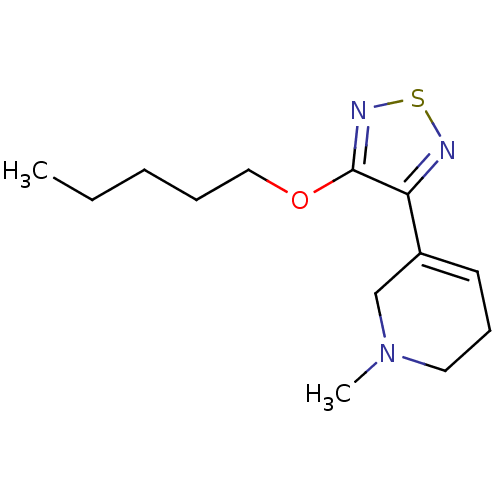
(1-Methyl-5-(4-pentyloxy-[1,2,5]thiadiazol-3-yl)-1,...)Show InChI InChI=1S/C13H21N3OS/c1-3-4-5-9-17-13-12(14-18-15-13)11-7-6-8-16(2)10-11/h7H,3-6,8-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072227

((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...)Show SMILES C(Oc1nsnc1[C@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3OS/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50213839
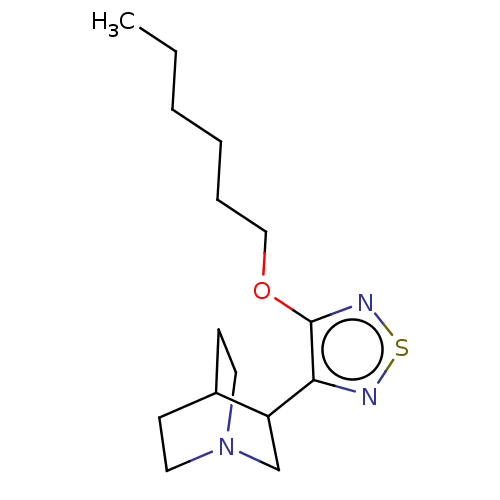
(CHEMBL98529)Show SMILES CCCCCCOc1nsnc1C1CN2CCC1CC2 |(14.95,-12.38,;14.22,-11.01,;12.68,-10.94,;11.97,-9.56,;10.43,-9.5,;9.72,-8.13,;8.18,-8.07,;7.36,-9.37,;7.92,-10.78,;6.73,-11.78,;5.43,-10.94,;5.8,-9.46,;4.83,-8.26,;3.54,-9.08,;2.17,-8.39,;3.19,-7.15,;3.83,-7.92,;4.77,-6.73,;3.41,-6.02,;2.13,-7.08,)| Show InChI InChI=1S/C15H25N3OS/c1-2-3-4-5-10-19-15-14(16-20-17-15)13-11-18-8-6-12(13)7-9-18/h12-13H,2-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072228
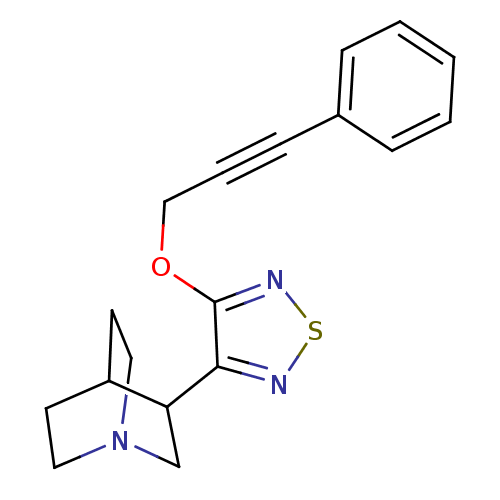
(3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3...)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccccc1 |(14.79,-5.45,;13.26,-5.32,;12.37,-6.6,;12.89,-8.07,;11.66,-9,;10.38,-8.12,;10.82,-6.64,;9.88,-5.4,;8.44,-5.96,;7.22,-5.01,;8.49,-3.99,;8.96,-4.86,;10.1,-3.86,;8.91,-2.91,;7.44,-3.72,;15.47,-6.86,;16.24,-8.18,;16.99,-9.52,;18.54,-9.52,;19.29,-10.85,;18.51,-12.2,;16.98,-12.2,;16.22,-10.85,)| Show InChI InChI=1S/C18H19N3OS/c1-2-5-14(6-3-1)7-4-12-22-18-17(19-23-20-18)16-13-21-10-8-15(16)9-11-21/h1-3,5-6,15-16H,8-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003365
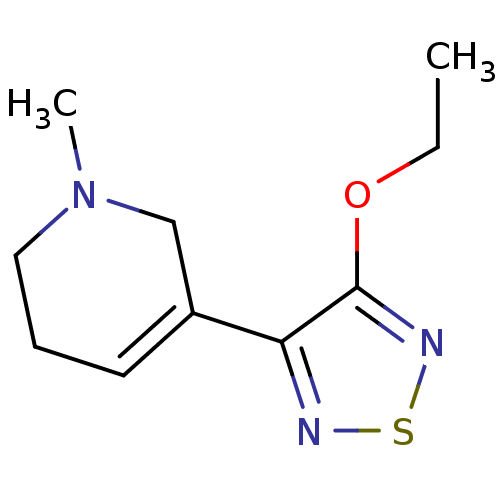
(3-(3-(ethoxy)-1,2,5-thiadiazol-4-yl)-1,2,5,6-tetra...)Show InChI InChI=1S/C10H15N3OS/c1-3-14-10-9(11-15-12-10)8-5-4-6-13(2)7-8/h5H,3-4,6-7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070700
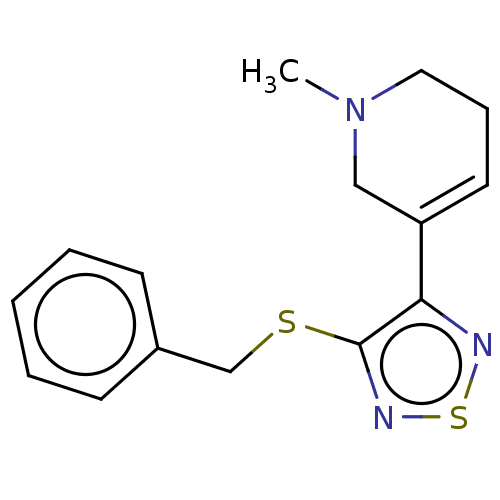
(CHEMBL100253)Show InChI InChI=1S/C25H39N5O8/c1-4-14(3)21(24(36)28-17(5-2)25(37)38)30-20(33)13-27-23(35)18(8-6-7-11-26)29-22(34)16-12-15(31)9-10-19(16)32/h9-10,12,14,17-18,21,31-32H,4-8,11,13,26H2,1-3H3,(H,27,35)(H,28,36)(H,29,34)(H,30,33)(H,37,38)/t14?,17?,18-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50213838
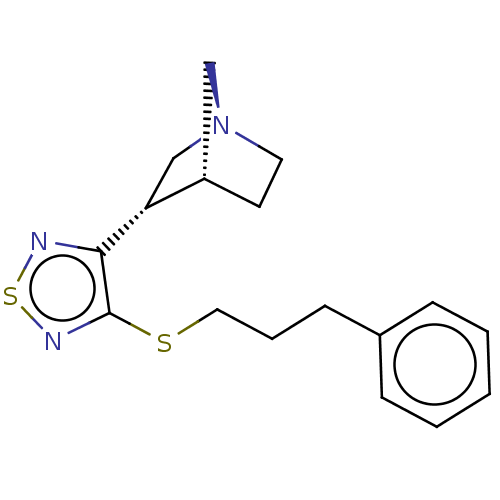
(CHEMBL98392)Show SMILES [H][C@@]12CC[N@@](C[C@H]1c1nsnc1SCCCc1ccccc1)C2 Show InChI InChI=1S/C17H21N3S2/c1-2-5-13(6-3-1)7-4-10-21-17-16(18-22-19-17)15-12-20-9-8-14(15)11-20/h1-3,5-6,14-15H,4,7-12H2/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070685

(CHEMBL98843)Show InChI InChI=1S/C32H45N5O8/c1-4-19(3)27(31(43)34-23(5-2)32(44)45)37-30(42)25(17-20-11-7-6-8-12-20)36-29(41)24(13-9-10-16-33)35-28(40)22-18-21(38)14-15-26(22)39/h6-8,11-12,14-15,18-19,23-25,27,38-39H,4-5,9-10,13,16-17,33H2,1-3H3,(H,34,43)(H,35,40)(H,36,41)(H,37,42)(H,44,45)/t19?,23?,24-,25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003356
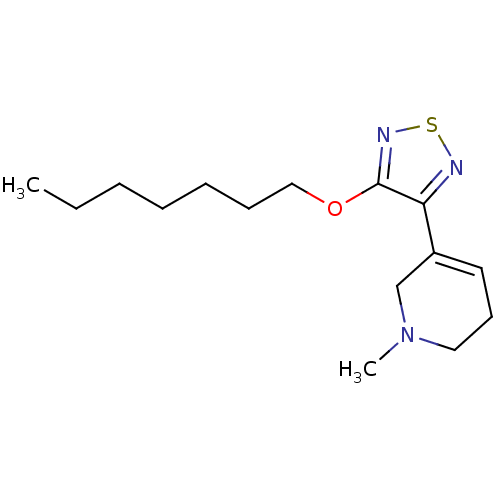
(5-(4-Heptyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,...)Show InChI InChI=1S/C15H25N3OS/c1-3-4-5-6-7-11-19-15-14(16-20-17-15)13-9-8-10-18(2)12-13/h9H,3-8,10-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070690

(CHEMBL328828)Show InChI InChI=1S/C28H43N7O8/c1-4-15(3)22(25(40)32-18(5-2)27(42)43)34-24(39)20-9-7-13-35(20)26(41)19(8-6-12-31-28(29)30)33-23(38)17-14-16(36)10-11-21(17)37/h10-11,14-15,18-20,22,36-37H,4-9,12-13H2,1-3H3,(H,32,40)(H,33,38)(H,34,39)(H,42,43)(H4,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072215
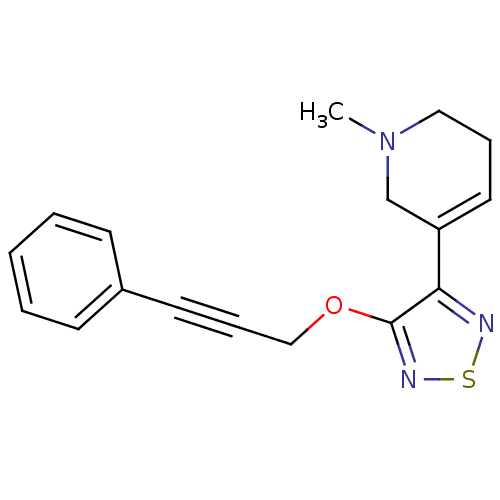
(1-Methyl-5-[4-(3-phenyl-prop-2-ynyloxy)-[1,2,5]thi...)Show InChI InChI=1S/C17H17N3OS/c1-20-11-5-10-15(13-20)16-17(19-22-18-16)21-12-6-9-14-7-3-2-4-8-14/h2-4,7-8,10H,5,11-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003359

(5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...)Show InChI InChI=1S/C14H23N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h8H,3-7,9-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070691

(CHEMBL98750)Show InChI InChI=1S/C28H45N5O8/c1-6-16(5)23(27(39)30-19(7-2)28(40)41)33-26(38)22(15(3)4)32-25(37)20(10-8-9-13-29)31-24(36)18-14-17(34)11-12-21(18)35/h11-12,14-16,19-20,22-23,34-35H,6-10,13,29H2,1-5H3,(H,30,39)(H,31,36)(H,32,37)(H,33,38)(H,40,41)/t16?,19?,20-,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072233

(1-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3...)Show InChI InChI=1S/C17H15N3OS/c1-2-5-12(6-3-1)7-4-8-21-16-15(18-22-19-16)17-11-20-9-13(17)14(17)10-20/h1-3,5-6,13-14H,8-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50213837
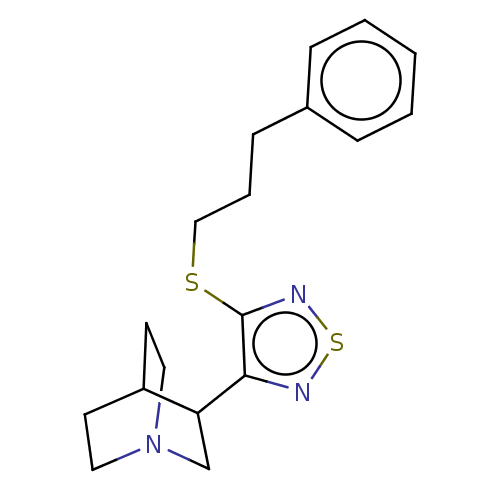
(CHEMBL318718)Show SMILES C(CSc1nsnc1C1CN2CCC1CC2)Cc1ccccc1 |(1.52,2.86,;.85,4.27,;-.68,4.4,;-1.57,3.12,;-1.05,1.66,;-2.27,.73,;-3.54,1.61,;-3.11,3.08,;-4.04,4.31,;-5.55,4.12,;-6.49,5.36,;-5.36,5.1,;-4.53,5.88,;-3.43,5.75,;-4.36,6.97,;-5.89,6.78,;3.06,2.73,;3.71,1.34,;5.25,1.21,;5.92,-.17,;5.03,-1.45,;3.49,-1.32,;2.84,.09,)| Show InChI InChI=1S/C18H23N3S2/c1-2-5-14(6-3-1)7-4-12-22-18-17(19-23-20-18)16-13-21-10-8-15(16)9-11-21/h1-3,5-6,15-16H,4,7-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070709
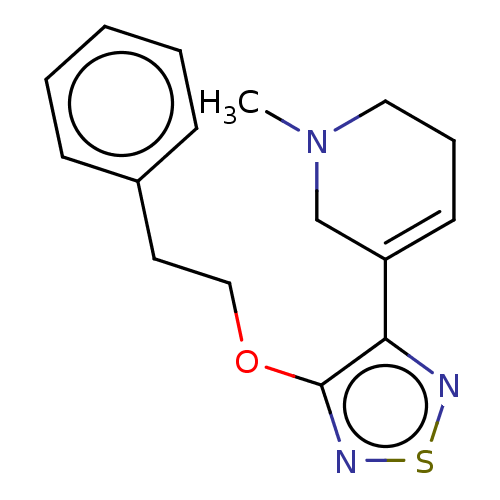
(CHEMBL102088)Show InChI InChI=1S/C28H45N5O8/c1-6-16(5)23(27(39)32-22(15(3)4)26(38)30-19(7-2)28(40)41)33-25(37)20(10-8-9-13-29)31-24(36)18-14-17(34)11-12-21(18)35/h11-12,14-16,19-20,22-23,34-35H,6-10,13,29H2,1-5H3,(H,30,38)(H,31,36)(H,32,39)(H,33,37)(H,40,41)/t16?,19?,20-,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072226

(3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3...)Show InChI InChI=1S/C17H17N3OS/c1-2-5-13(6-3-1)7-4-10-21-17-16(18-22-19-17)15-12-20-9-8-14(15)11-20/h1-3,5-6,14-15H,8-12H2/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072234
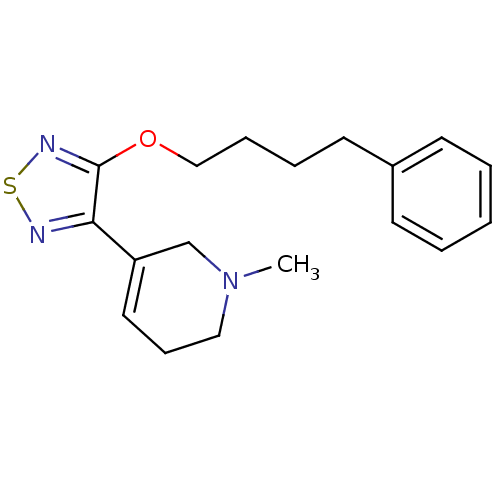
(1-Methyl-5-[4-(4-phenyl-butoxy)-[1,2,5]thiadiazol-...)Show InChI InChI=1S/C18H23N3OS/c1-21-12-7-11-16(14-21)17-18(20-23-19-17)22-13-6-5-10-15-8-3-2-4-9-15/h2-4,8-9,11H,5-7,10,12-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003361
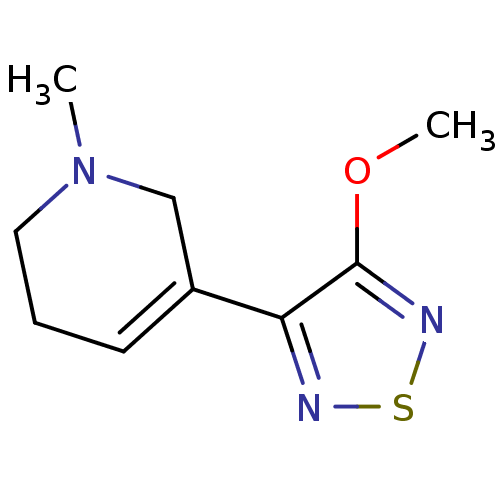
(5-(4-Methoxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2,...)Show InChI InChI=1S/C9H13N3OS/c1-12-5-3-4-7(6-12)8-9(13-2)11-14-10-8/h4H,3,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50004656

((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...)Show InChI InChI=1S/C6H14N2O2/c1-8(2,3)4-5-10-6(7)9/h4-5H2,1-3H3,(H-,7,9)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070718
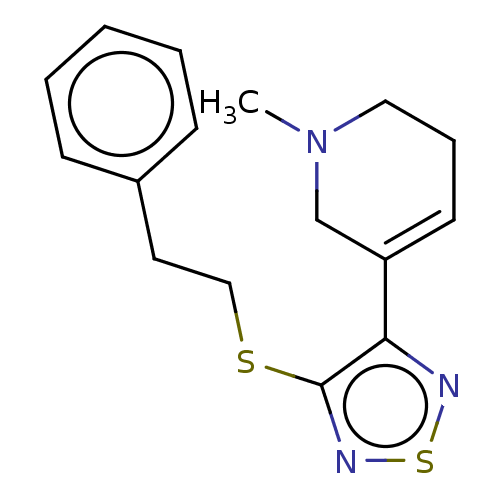
(CHEMBL98759)Show InChI InChI=1S/C29H48N8O8/c1-4-16(3)23(27(43)36-21(10-8-14-33-29(31)32)25(41)34-19(5-2)28(44)45)37-26(42)20(9-6-7-13-30)35-24(40)18-15-17(38)11-12-22(18)39/h11-12,15-16,19-21,23,38-39H,4-10,13-14,30H2,1-3H3,(H,34,41)(H,35,40)(H,36,43)(H,37,42)(H,44,45)(H4,31,32,33)/t16?,19?,20-,21-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM46858
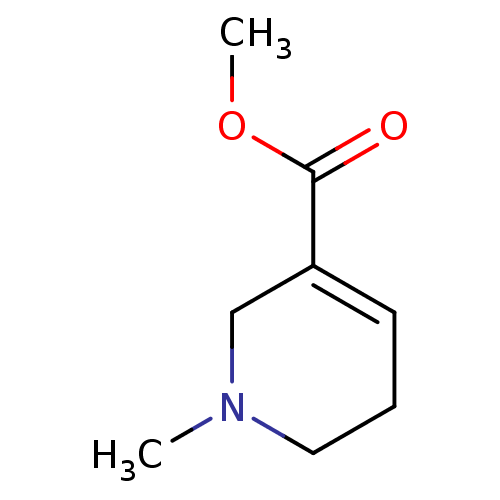
(1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...)Show InChI InChI=1S/C8H13NO2/c1-9-5-3-4-7(6-9)8(10)11-2/h4H,3,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50006588
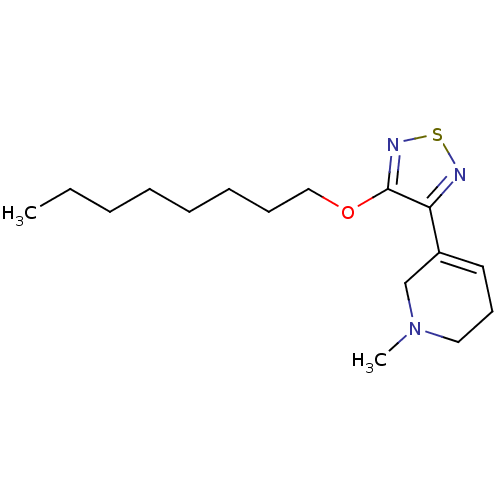
(1-Methyl-5-(4-octyloxy-[1,2,5]thiadiazol-3-yl)-1,2...)Show InChI InChI=1S/C16H27N3OS/c1-3-4-5-6-7-8-12-20-16-15(17-21-18-16)14-10-9-11-19(2)13-14/h10H,3-9,11-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50071866
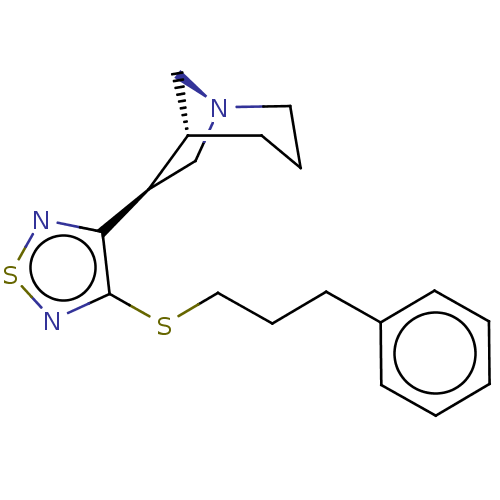
(CHEMBL98757)Show SMILES [H][C@]12C[N@](C[C@H]1c1nsnc1SCCCc1ccccc1)CCC2 Show InChI InChI=1S/C27H31N3O2/c1-17-12-21(31)13-18(2)22(17)16-24(28)27(32)30-26-10-11-29-25-9-8-20(15-23(25)26)14-19-6-4-3-5-7-19/h3-9,12-13,15,24,26,29,31H,10-11,14,16,28H2,1-2H3,(H,30,32)/t24-,26?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50366538

(CHEMBL1169506)Show SMILES C(Oc1nsnc1[C@H]1CN2CC[C@@H]1C2)C#Cc1ccccc1 |r,TLB:6:7:11.10:13| Show InChI InChI=1S/C17H17N3OS/c1-2-5-13(6-3-1)7-4-10-21-17-16(18-22-19-17)15-12-20-9-8-14(15)11-20/h1-3,5-6,14-15H,8-12H2/t14-,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072229
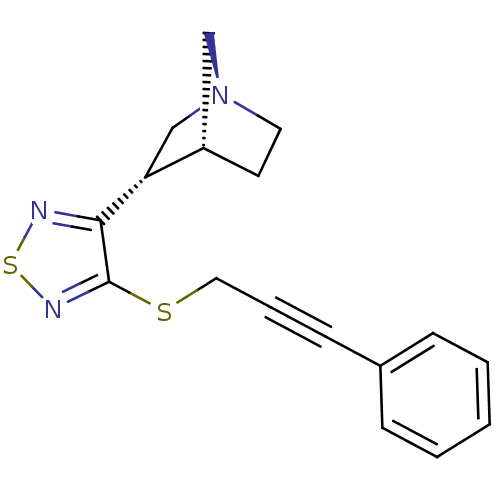
((1R,3S,4R)-3-[4-(3-Phenyl-prop-2-ynylsulfanyl)-[1,...)Show SMILES C(Sc1nsnc1[C@@H]1C[N@@]2CC[C@H]1C2)C#Cc1ccccc1 Show InChI InChI=1S/C17H17N3S2/c1-2-5-13(6-3-1)7-4-10-21-17-16(18-22-19-17)15-12-20-9-8-14(15)11-20/h1-3,5-6,14-15H,8-12H2/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50213836
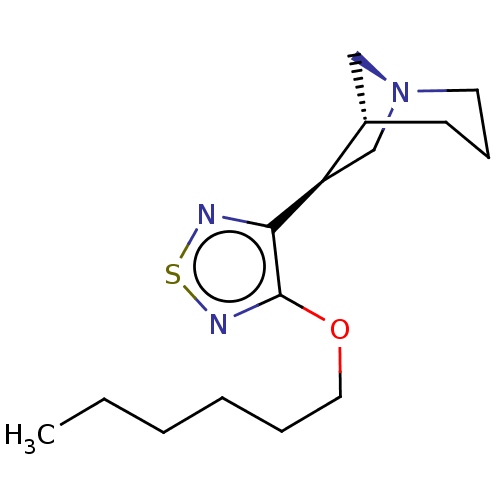
(CHEMBL99424)Show InChI InChI=1S/C15H25N3OS/c1-2-3-4-5-9-19-15-14(16-20-17-15)13-11-18-8-6-7-12(13)10-18/h12-13H,2-11H2,1H3/t12-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072220
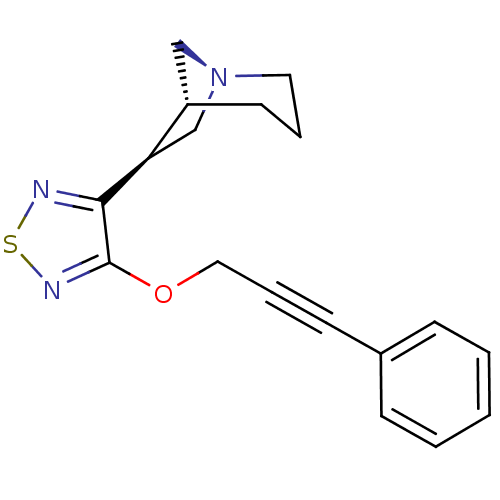
((1R,5R,6S)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...)Show SMILES C(Oc1nsnc1[C@@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3OS/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072220

((1R,5R,6S)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...)Show SMILES C(Oc1nsnc1[C@@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3OS/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50008072
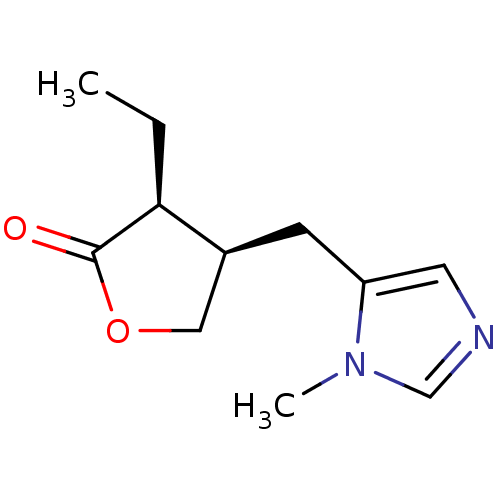
((+)-pilocarpine | (3S,4R)-3-ethyl-4-[(1-methyl-1H-...)Show InChI InChI=1S/C11H16N2O2/c1-3-10-8(6-15-11(10)14)4-9-5-12-7-13(9)2/h5,7-8,10H,3-4,6H2,1-2H3/t8-,10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50065223

(1-Aza-bicyclo[2.2.1]heptan-3-one O-[3-(3-methoxy-p...)Show InChI InChI=1S/C16H18N2O2/c1-19-15-6-2-4-13(10-15)5-3-9-20-17-16-12-18-8-7-14(16)11-18/h2,4,6,10,14H,7-9,11-12H2,1H3/b17-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072224
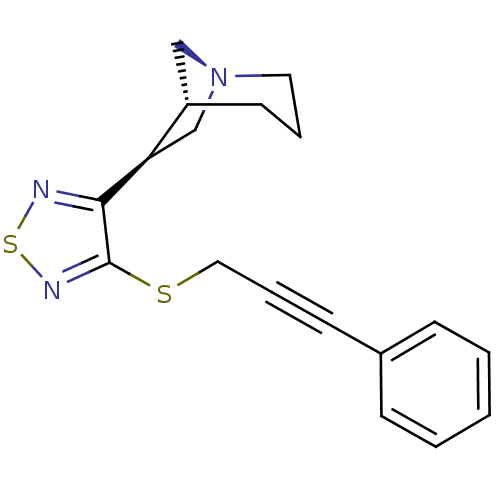
((1R,5R,6S)-6-[4-(3-Phenyl-prop-2-ynylsulfanyl)-[1,...)Show SMILES C(Sc1nsnc1[C@@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3S2/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 363 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50072229

((1R,3S,4R)-3-[4-(3-Phenyl-prop-2-ynylsulfanyl)-[1,...)Show SMILES C(Sc1nsnc1[C@@H]1C[N@@]2CC[C@H]1C2)C#Cc1ccccc1 Show InChI InChI=1S/C17H17N3S2/c1-2-5-13(6-3-1)7-4-10-21-17-16(18-22-19-17)15-12-20-9-8-14(15)11-20/h1-3,5-6,14-15H,8-12H2/t14-,15+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 139 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50008072

((+)-pilocarpine | (3S,4R)-3-ethyl-4-[(1-methyl-1H-...)Show InChI InChI=1S/C11H16N2O2/c1-3-10-8(6-15-11(10)14)4-9-5-12-7-13(9)2/h5,7-8,10H,3-4,6H2,1-2H3/t8-,10-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50072227

((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...)Show SMILES C(Oc1nsnc1[C@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3OS/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50072214

((R)-3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiaz...)Show InChI InChI=1S/C17H17N3OS/c1-2-5-13(6-3-1)7-4-10-21-17-16(18-22-19-17)15-12-20-9-8-14(15)11-20/h1-3,5-6,14-15H,8-12H2/t14?,15-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50366538

(CHEMBL1169506)Show SMILES C(Oc1nsnc1[C@H]1CN2CC[C@@H]1C2)C#Cc1ccccc1 |r,TLB:6:7:11.10:13| Show InChI InChI=1S/C17H17N3OS/c1-2-5-13(6-3-1)7-4-10-21-17-16(18-22-19-17)15-12-20-9-8-14(15)11-20/h1-3,5-6,14-15H,8-12H2/t14-,15+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 159 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50004656

((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...)Show InChI InChI=1S/C6H14N2O2/c1-8(2,3)4-5-10-6(7)9/h4-5H2,1-3H3,(H-,7,9)/p+1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.81E+3 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM46858

(1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...)Show InChI InChI=1S/C8H13NO2/c1-9-5-3-4-7(6-9)8(10)11-2/h4H,3,5-6H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50072220

((1R,5R,6S)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...)Show SMILES C(Oc1nsnc1[C@@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3OS/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 227 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50072220

((1R,5R,6S)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...)Show SMILES C(Oc1nsnc1[C@@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3OS/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50072229

((1R,3S,4R)-3-[4-(3-Phenyl-prop-2-ynylsulfanyl)-[1,...)Show SMILES C(Sc1nsnc1[C@@H]1C[N@@]2CC[C@H]1C2)C#Cc1ccccc1 Show InChI InChI=1S/C17H17N3S2/c1-2-5-13(6-3-1)7-4-10-21-17-16(18-22-19-17)15-12-20-9-8-14(15)11-20/h1-3,5-6,14-15H,8-12H2/t14-,15+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >100 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50072227

((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...)Show SMILES C(Oc1nsnc1[C@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3OS/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50072215

(1-Methyl-5-[4-(3-phenyl-prop-2-ynyloxy)-[1,2,5]thi...)Show InChI InChI=1S/C17H17N3OS/c1-20-11-5-10-15(13-20)16-17(19-22-18-16)21-12-6-9-14-7-3-2-4-8-14/h2-4,7-8,10H,5,11-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50072225

((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynylsulfanyl)-[1,...)Show SMILES C(Sc1nsnc1[C@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3S2/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50072226

(3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3...)Show InChI InChI=1S/C17H17N3OS/c1-2-5-13(6-3-1)7-4-10-21-17-16(18-22-19-17)15-12-20-9-8-14(15)11-20/h1-3,5-6,14-15H,8-12H2/t14-,15+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 67 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50072228

(3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3...)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccccc1 |(14.79,-5.45,;13.26,-5.32,;12.37,-6.6,;12.89,-8.07,;11.66,-9,;10.38,-8.12,;10.82,-6.64,;9.88,-5.4,;8.44,-5.96,;7.22,-5.01,;8.49,-3.99,;8.96,-4.86,;10.1,-3.86,;8.91,-2.91,;7.44,-3.72,;15.47,-6.86,;16.24,-8.18,;16.99,-9.52,;18.54,-9.52,;19.29,-10.85,;18.51,-12.2,;16.98,-12.2,;16.22,-10.85,)| Show InChI InChI=1S/C18H19N3OS/c1-2-5-14(6-3-1)7-4-12-22-18-17(19-23-20-18)16-13-21-10-8-15(16)9-11-21/h1-3,5-6,15-16H,8-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50072233

(1-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3...)Show InChI InChI=1S/C17H15N3OS/c1-2-5-12(6-3-1)7-4-8-21-16-15(18-22-19-16)17-11-20-9-13(17)14(17)10-20/h1-3,5-6,13-14H,8-11H2 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 71 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50072233

(1-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3...)Show InChI InChI=1S/C17H15N3OS/c1-2-5-12(6-3-1)7-4-8-21-16-15(18-22-19-16)17-11-20-9-13(17)14(17)10-20/h1-3,5-6,13-14H,8-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 779 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM46858

(1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...)Show InChI InChI=1S/C8H13NO2/c1-9-5-3-4-7(6-9)8(10)11-2/h4H,3,5-6H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50065223

(1-Aza-bicyclo[2.2.1]heptan-3-one O-[3-(3-methoxy-p...)Show InChI InChI=1S/C16H18N2O2/c1-19-15-6-2-4-13(10-15)5-3-9-20-17-16-12-18-8-7-14(16)11-18/h2,4,6,10,14H,7-9,11-12H2,1H3/b17-16+ | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50065223

(1-Aza-bicyclo[2.2.1]heptan-3-one O-[3-(3-methoxy-p...)Show InChI InChI=1S/C16H18N2O2/c1-19-15-6-2-4-13(10-15)5-3-9-20-17-16-12-18-8-7-14(16)11-18/h2,4,6,10,14H,7-9,11-12H2,1H3/b17-16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50008072

((+)-pilocarpine | (3S,4R)-3-ethyl-4-[(1-methyl-1H-...)Show InChI InChI=1S/C11H16N2O2/c1-3-10-8(6-15-11(10)14)4-9-5-12-7-13(9)2/h5,7-8,10H,3-4,6H2,1-2H3/t8-,10-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 8.79E+3 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50072227

((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...)Show SMILES C(Oc1nsnc1[C@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3OS/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50072215

(1-Methyl-5-[4-(3-phenyl-prop-2-ynyloxy)-[1,2,5]thi...)Show InChI InChI=1S/C17H17N3OS/c1-20-11-5-10-15(13-20)16-17(19-22-18-16)21-12-6-9-14-7-3-2-4-8-14/h2-4,7-8,10H,5,11-13H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50072226

(3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3...)Show InChI InChI=1S/C17H17N3OS/c1-2-5-13(6-3-1)7-4-10-21-17-16(18-22-19-17)15-12-20-9-8-14(15)11-20/h1-3,5-6,14-15H,8-12H2/t14-,15+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50072225

((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynylsulfanyl)-[1,...)Show SMILES C(Sc1nsnc1[C@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3S2/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Effective concentration required for stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50072214

((R)-3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiaz...)Show InChI InChI=1S/C17H17N3OS/c1-2-5-13(6-3-1)7-4-10-21-17-16(18-22-19-17)15-12-20-9-8-14(15)11-20/h1-3,5-6,14-15H,8-12H2/t14?,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50072224

((1R,5R,6S)-6-[4-(3-Phenyl-prop-2-ynylsulfanyl)-[1,...)Show SMILES C(Sc1nsnc1[C@@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3S2/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16+/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50004656

((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...)Show InChI InChI=1S/C6H14N2O2/c1-8(2,3)4-5-10-6(7)9/h4-5H2,1-3H3,(H-,7,9)/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50072228

(3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3...)Show SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccccc1 |(14.79,-5.45,;13.26,-5.32,;12.37,-6.6,;12.89,-8.07,;11.66,-9,;10.38,-8.12,;10.82,-6.64,;9.88,-5.4,;8.44,-5.96,;7.22,-5.01,;8.49,-3.99,;8.96,-4.86,;10.1,-3.86,;8.91,-2.91,;7.44,-3.72,;15.47,-6.86,;16.24,-8.18,;16.99,-9.52,;18.54,-9.52,;19.29,-10.85,;18.51,-12.2,;16.98,-12.2,;16.22,-10.85,)| Show InChI InChI=1S/C18H19N3OS/c1-2-5-14(6-3-1)7-4-12-22-18-17(19-23-20-18)16-13-21-10-8-15(16)9-11-21/h1-3,5-6,15-16H,8-13H2 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50072224

((1R,5R,6S)-6-[4-(3-Phenyl-prop-2-ynylsulfanyl)-[1,...)Show SMILES C(Sc1nsnc1[C@@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3S2/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50072220

((1R,5R,6S)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...)Show SMILES C(Oc1nsnc1[C@@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3OS/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 227 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50366538

(CHEMBL1169506)Show SMILES C(Oc1nsnc1[C@H]1CN2CC[C@@H]1C2)C#Cc1ccccc1 |r,TLB:6:7:11.10:13| Show InChI InChI=1S/C17H17N3OS/c1-2-5-13(6-3-1)7-4-10-21-17-16(18-22-19-17)15-12-20-9-8-14(15)11-20/h1-3,5-6,14-15H,8-12H2/t14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data