

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072583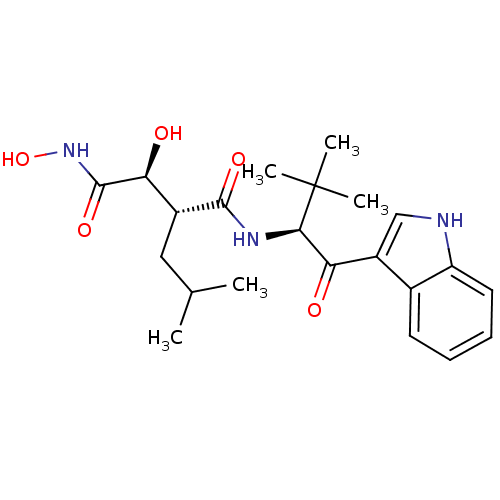 ((2R,3S)-2,N*1*-Dihydroxy-N*4*-[(S)-1-(1H-indole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072583 ((2R,3S)-2,N*1*-Dihydroxy-N*4*-[(S)-1-(1H-indole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072583 ((2R,3S)-2,N*1*-Dihydroxy-N*4*-[(S)-1-(1H-indole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072564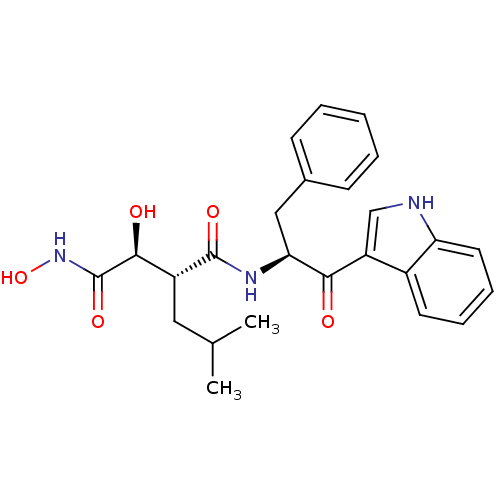 ((2R,3S)-N*4*-[(S)-1-Benzyl-2-(1H-indol-3-yl)-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50063918 ((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072563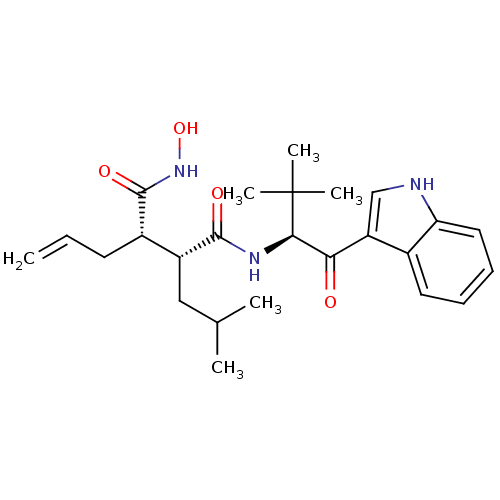 ((2R,3S)-2-Allyl-N*1*-hydroxy-N*4*-[(S)-1-(1H-indol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063917 ((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072575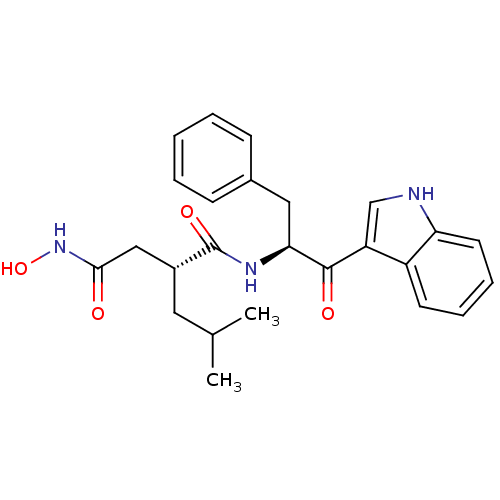 ((R)-N*1*-[(S)-1-Benzyl-2-(1H-indol-3-yl)-2-oxo-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50063917 ((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072566 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-(1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072564 ((2R,3S)-N*4*-[(S)-1-Benzyl-2-(1H-indol-3-yl)-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072566 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-(1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072582 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-oxo-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072583 ((2R,3S)-2,N*1*-Dihydroxy-N*4*-[(S)-1-(1H-indole-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072563 ((2R,3S)-2-Allyl-N*1*-hydroxy-N*4*-[(S)-1-(1H-indol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072582 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-oxo-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072563 ((2R,3S)-2-Allyl-N*1*-hydroxy-N*4*-[(S)-1-(1H-indol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50063918 ((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072572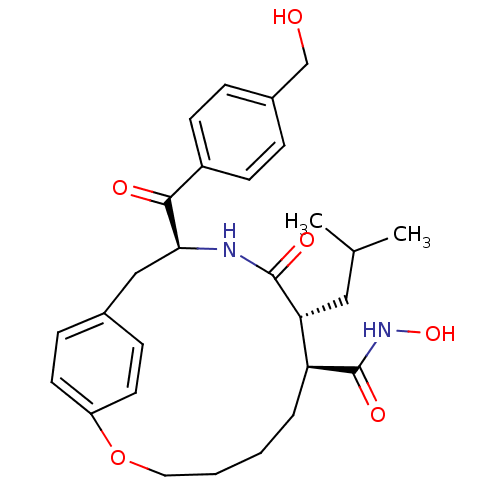 ((7S,8R,11S)-11-(4-Hydroxymethyl-benzoyl)-8-isobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072563 ((2R,3S)-2-Allyl-N*1*-hydroxy-N*4*-[(S)-1-(1H-indol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50071263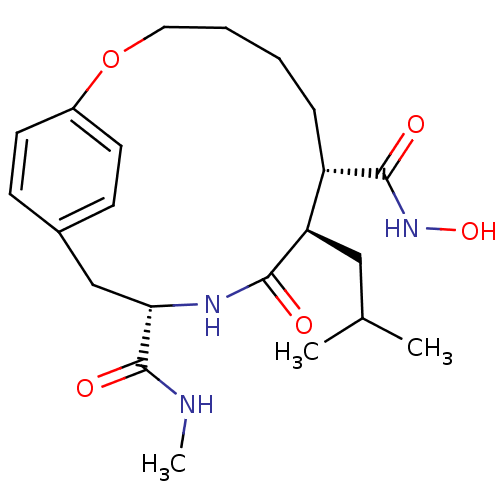 ((7S,8R,11S)-8-Isobutyl-9-oxo-2-oxa-10-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072582 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-oxo-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50070453 ((2R,3S)-2-Allyl-N*1*-hydroxy-3-isobutyl-N*4*-((S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50071263 ((7S,8R,11S)-8-Isobutyl-9-oxo-2-oxa-10-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072569 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxazol-2-yl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072574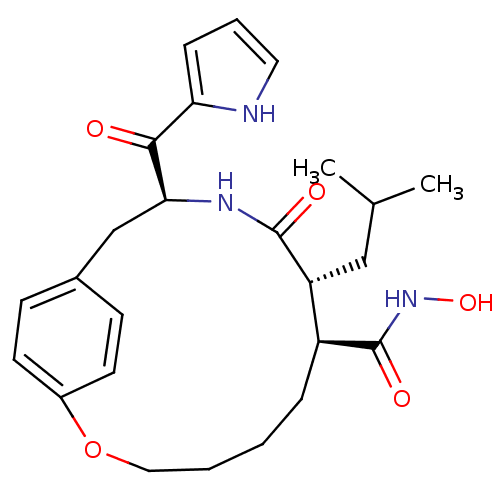 ((7S,8R,11S)-8-Isobutyl-9-oxo-11-(1H-pyrrole-2-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063918 ((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50071262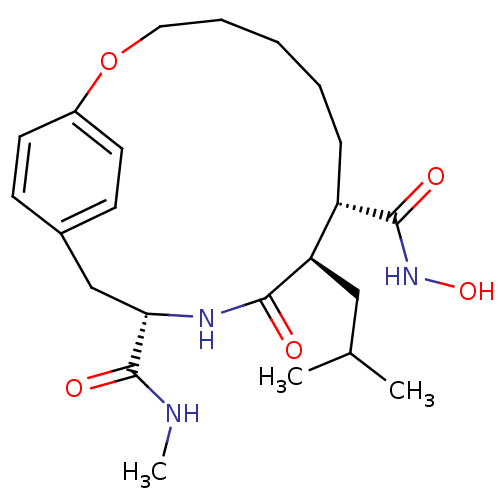 ((8S,9R,12S)-9-Isobutyl-10-oxo-2-oxa-11-aza-bicyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072579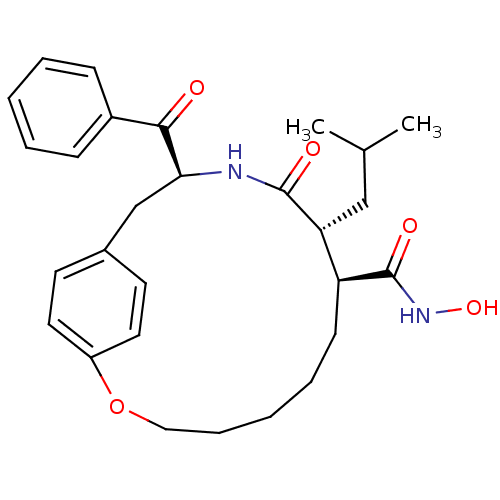 ((8S,9R,12S)-12-Benzoyl-9-isobutyl-10-oxo-2-oxa-11-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50070453 ((2R,3S)-2-Allyl-N*1*-hydroxy-3-isobutyl-N*4*-((S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072566 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-(1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072566 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-(1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50071262 ((8S,9R,12S)-9-Isobutyl-10-oxo-2-oxa-11-aza-bicyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50063918 ((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072582 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-oxo-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50071262 ((8S,9R,12S)-9-Isobutyl-10-oxo-2-oxa-11-aza-bicyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072569 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxazol-2-yl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072580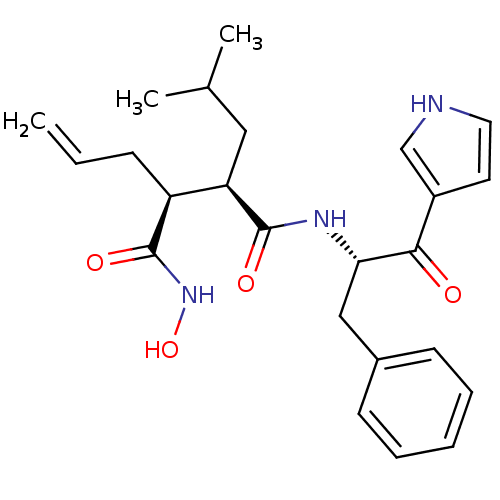 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-oxo-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072579 ((8S,9R,12S)-12-Benzoyl-9-isobutyl-10-oxo-2-oxa-11-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072568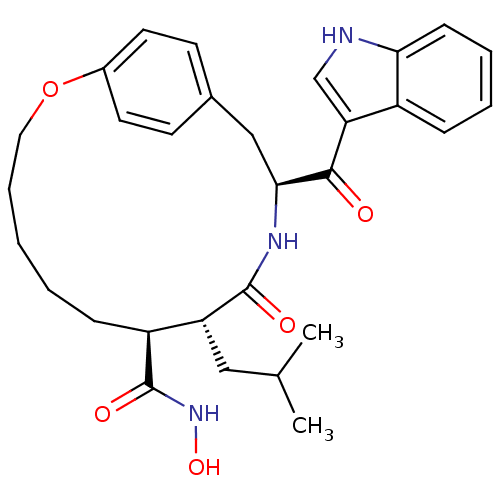 ((8S,9R,12S)-12-(1H-Indole-3-carbonyl)-9-isobutyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072571 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072567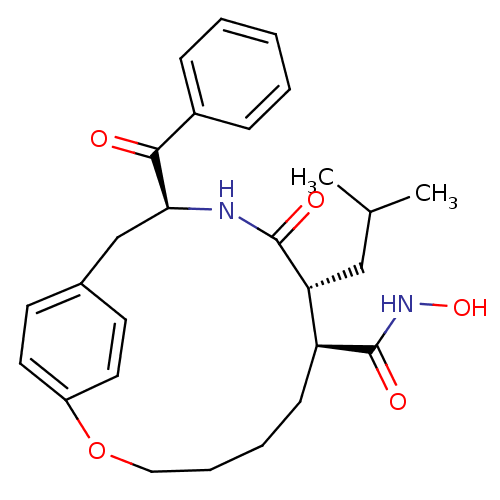 ((7S,8R,11S)-11-Benzoyl-8-isobutyl-9-oxo-2-oxa-10-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072578 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072572 ((7S,8R,11S)-11-(4-Hydroxymethyl-benzoyl)-8-isobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072578 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072579 ((8S,9R,12S)-12-Benzoyl-9-isobutyl-10-oxo-2-oxa-11-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50071263 ((7S,8R,11S)-8-Isobutyl-9-oxo-2-oxa-10-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072569 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxazol-2-yl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072574 ((7S,8R,11S)-8-Isobutyl-9-oxo-11-(1H-pyrrole-2-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072575 ((R)-N*1*-[(S)-1-Benzyl-2-(1H-indol-3-yl)-2-oxo-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072578 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072581 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-(1-methyl-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072568 ((8S,9R,12S)-12-(1H-Indole-3-carbonyl)-9-isobutyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072564 ((2R,3S)-N*4*-[(S)-1-Benzyl-2-(1H-indol-3-yl)-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072572 ((7S,8R,11S)-11-(4-Hydroxymethyl-benzoyl)-8-isobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072572 ((7S,8R,11S)-11-(4-Hydroxymethyl-benzoyl)-8-isobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072568 ((8S,9R,12S)-12-(1H-Indole-3-carbonyl)-9-isobutyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072575 ((R)-N*1*-[(S)-1-Benzyl-2-(1H-indol-3-yl)-2-oxo-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072575 ((R)-N*1*-[(S)-1-Benzyl-2-(1H-indol-3-yl)-2-oxo-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50071263 ((7S,8R,11S)-8-Isobutyl-9-oxo-2-oxa-10-aza-bicyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072576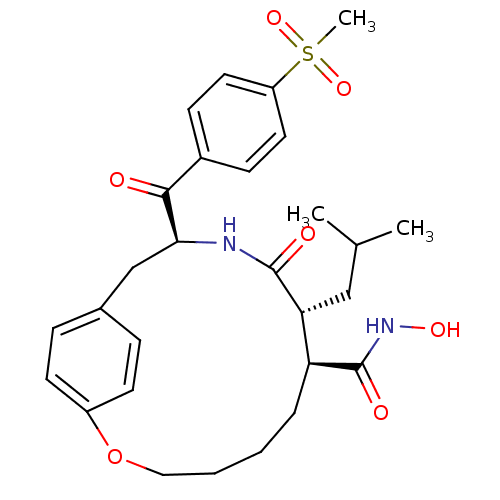 ((7S,8R,11S)-8-Isobutyl-11-(4-methanesulfonyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50071262 ((8S,9R,12S)-9-Isobutyl-10-oxo-2-oxa-11-aza-bicyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072568 ((8S,9R,12S)-12-(1H-Indole-3-carbonyl)-9-isobutyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072580 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-oxo-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072571 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072580 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-oxo-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072574 ((7S,8R,11S)-8-Isobutyl-9-oxo-11-(1H-pyrrole-2-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072574 ((7S,8R,11S)-8-Isobutyl-9-oxo-11-(1H-pyrrole-2-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072569 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxazol-2-yl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072564 ((2R,3S)-N*4*-[(S)-1-Benzyl-2-(1H-indol-3-yl)-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072580 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-oxo-2-(1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072576 ((7S,8R,11S)-8-Isobutyl-11-(4-methanesulfonyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072577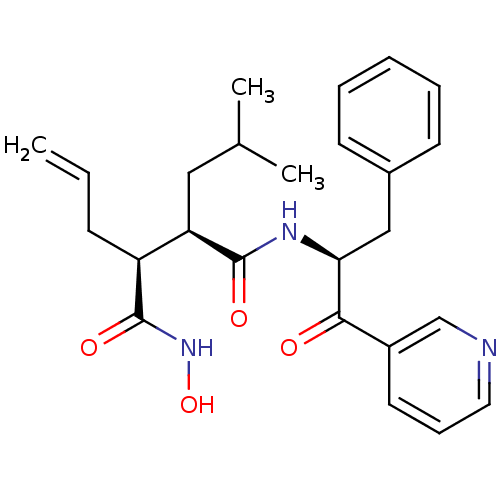 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072577 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072567 ((7S,8R,11S)-11-Benzoyl-8-isobutyl-9-oxo-2-oxa-10-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072579 ((8S,9R,12S)-12-Benzoyl-9-isobutyl-10-oxo-2-oxa-11-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072567 ((7S,8R,11S)-11-Benzoyl-8-isobutyl-9-oxo-2-oxa-10-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072576 ((7S,8R,11S)-8-Isobutyl-11-(4-methanesulfonyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072578 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-phenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50063917 ((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072577 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50070453 ((2R,3S)-2-Allyl-N*1*-hydroxy-3-isobutyl-N*4*-((S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50063917 ((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50070453 ((2R,3S)-2-Allyl-N*1*-hydroxy-3-isobutyl-N*4*-((S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072567 ((7S,8R,11S)-11-Benzoyl-8-isobutyl-9-oxo-2-oxa-10-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072571 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072577 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072570 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-propyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072570 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-propyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072581 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-(1-methyl-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072581 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-(1-methyl-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072562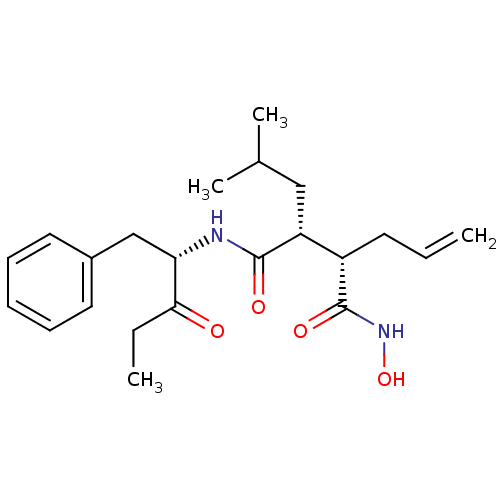 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-butyl)-N*...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072581 ((2R,3S)-2-Allyl-N*4*-[(S)-1-benzyl-2-(1-methyl-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072562 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-butyl)-N*...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072584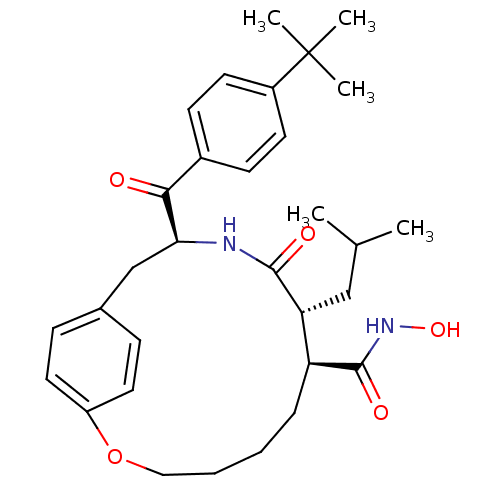 ((7S,8R,11S)-11-(4-tert-Butyl-benzoyl)-8-isobutyl-9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072562 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-butyl)-N*...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072570 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-propyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072565 ((2R,3S)-2-Allyl-N*4*-[(S)-2-(1H-benzoimidazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072576 ((7S,8R,11S)-8-Isobutyl-11-(4-methanesulfonyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072565 ((2R,3S)-2-Allyl-N*4*-[(S)-2-(1H-benzoimidazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072562 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-butyl)-N*...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072584 ((7S,8R,11S)-11-(4-tert-Butyl-benzoyl)-8-isobutyl-9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072584 ((7S,8R,11S)-11-(4-tert-Butyl-benzoyl)-8-isobutyl-9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072565 ((2R,3S)-2-Allyl-N*4*-[(S)-2-(1H-benzoimidazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 2 isolated from human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072565 ((2R,3S)-2-Allyl-N*4*-[(S)-2-(1H-benzoimidazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50072573 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-thiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against human recombinant matrix metalloproteinase 7 | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50072584 ((7S,8R,11S)-11-(4-tert-Butyl-benzoyl)-8-isobutyl-9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase 1 isolated from the culture medium of human skin fibroblasts induced with PMA | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50072573 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-thiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibition of human recombinant matrix metalloproteinase 3 (MMP3) | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50072573 ((2R,3S)-2-Allyl-N*4*-((S)-1-benzyl-2-oxo-2-thiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibition of matrix metalloproteinase 2 (MMP2) of human HT-1080 fibrosarcoma cells induced with TNF | Bioorg Med Chem Lett 8: 3251-6 (1999) BindingDB Entry DOI: 10.7270/Q289152J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||