

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50073120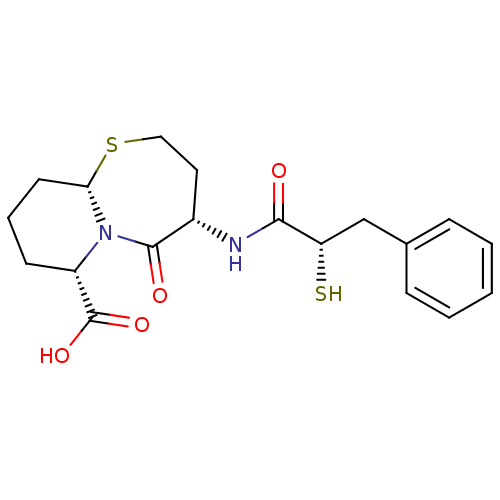 ((4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) isolated from rabbit lung extract using hippuryl-L-histidyl-L-leucine (HHL... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50073118 (CHEMBL319237 | [(S)-8-((S)-2-Mercapto-3-phenyl-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) isolated from rabbit lung extract using hippuryl-L-histidyl-L-leucine (HHL... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50073120 ((4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified rat kidney neutral endopeptidase (NEP) using a fluorometric assay with dansyl-Gly-Phe-Arg as the substr... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048506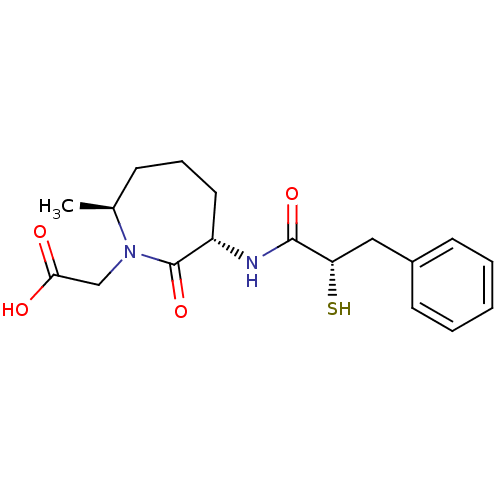 (CHEMBL104054 | [(3S,7S)-3-((S)-2-Mercapto-3-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) isolated from rabbit lung extract using hippuryl-L-histidyl-L-leucine (HHL... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50073121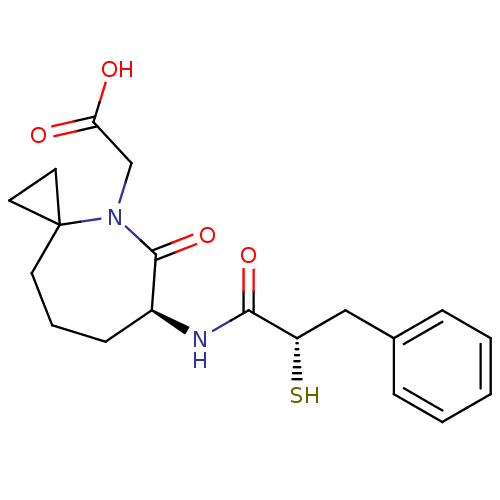 (CHEMBL430484 | [(S)-6-((S)-2-Mercapto-3-phenyl-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) isolated from rabbit lung extract using hippuryl-L-histidyl-L-leucine (HHL... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50073119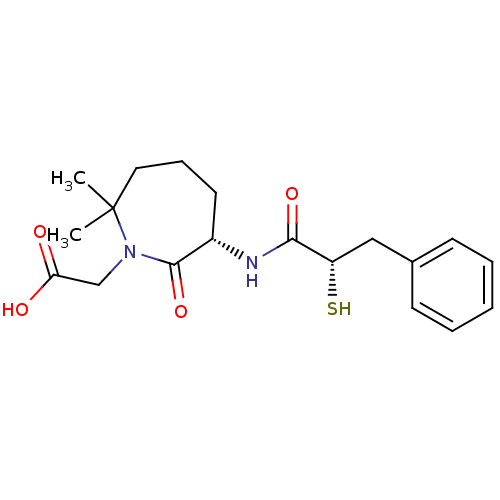 (CHEMBL107747 | Gemopatrilat | [(S)-6-((S)-2-Mercap...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) isolated from rabbit lung extract using hippuryl-L-histidyl-L-leucine (HHL... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048517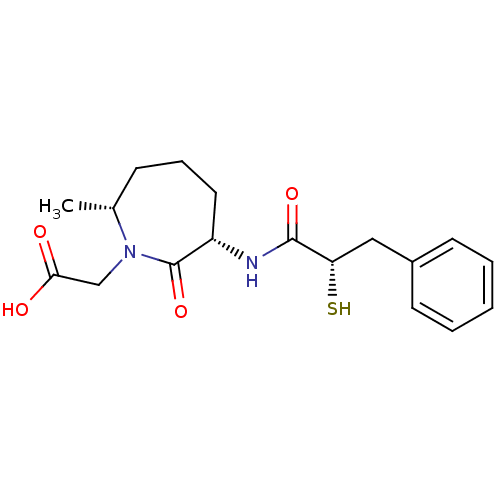 (CHEMBL318096 | [(3S,7R)-3-((S)-2-Mercapto-3-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) isolated from rabbit lung extract using hippuryl-L-histidyl-L-leucine (HHL... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048507 (CHEMBL299169 | [(S)-3-((S)-2-Mercapto-3-phenyl-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) isolated from rabbit lung extract using hippuryl-L-histidyl-L-leucine (HHL... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50073122 (CHEMBL107275 | [(S)-7-((S)-2-Mercapto-3-phenyl-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme (ACE) isolated from rabbit lung extract using hippuryl-L-histidyl-L-leucine (HHL... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048506 (CHEMBL104054 | [(3S,7S)-3-((S)-2-Mercapto-3-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified rat kidney neutral endopeptidase (NEP) using a fluorometric assay with dansyl-Gly-Phe-Arg as the substr... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50073119 (CHEMBL107747 | Gemopatrilat | [(S)-6-((S)-2-Mercap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified rat kidney neutral endopeptidase (NEP) using a fluorometric assay with dansyl-Gly-Phe-Arg as the substr... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048507 (CHEMBL299169 | [(S)-3-((S)-2-Mercapto-3-phenyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified rat kidney neutral endopeptidase (NEP) using a fluorometric assay with dansyl-Gly-Phe-Arg as the substr... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048517 (CHEMBL318096 | [(3S,7R)-3-((S)-2-Mercapto-3-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified rat kidney neutral endopeptidase (NEP) using a fluorometric assay with dansyl-Gly-Phe-Arg as the substr... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50073121 (CHEMBL430484 | [(S)-6-((S)-2-Mercapto-3-phenyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 377 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified rat kidney neutral endopeptidase (NEP) using a fluorometric assay with dansyl-Gly-Phe-Arg as the substr... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50073118 (CHEMBL319237 | [(S)-8-((S)-2-Mercapto-3-phenyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 426 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified rat kidney neutral endopeptidase (NEP) using a fluorometric assay with dansyl-Gly-Phe-Arg as the substr... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50073122 (CHEMBL107275 | [(S)-7-((S)-2-Mercapto-3-phenyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 641 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against purified rat kidney neutral endopeptidase (NEP) using a fluorometric assay with dansyl-Gly-Phe-Arg as the substr... | J Med Chem 42: 305-11 (1999) Article DOI: 10.1021/jm980542f BindingDB Entry DOI: 10.7270/Q2DV1J2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||