

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Coagulation factor XI | ||
| Ligand | BDBM12975 | ||
| Substrate/Competitor | BDBM12947 | ||
| Meas. Tech. | Enzyme Inhibition Assay | ||
| IC50 | 29±n/a nM | ||
| Citation |  Lin, J; Deng, H; Jin, L; Pandey, P; Quinn, J; Cantin, S; Rynkiewicz, MJ; Gorga, JC; Bibbins, F; Celatka, CA; Nagafuji, P; Bannister, TD; Meyers, HV; Babine, RE; Hayward, NJ; Weaver, D; Benjamin, H; Stassen, F; Abdel-Meguid, SS; Strickler, JE Design, synthesis, and biological evaluation of peptidomimetic inhibitors of factor XIa as novel anticoagulants. J Med Chem49:7781-91 (2006) [PubMed] Article Lin, J; Deng, H; Jin, L; Pandey, P; Quinn, J; Cantin, S; Rynkiewicz, MJ; Gorga, JC; Bibbins, F; Celatka, CA; Nagafuji, P; Bannister, TD; Meyers, HV; Babine, RE; Hayward, NJ; Weaver, D; Benjamin, H; Stassen, F; Abdel-Meguid, SS; Strickler, JE Design, synthesis, and biological evaluation of peptidomimetic inhibitors of factor XIa as novel anticoagulants. J Med Chem49:7781-91 (2006) [PubMed] Article | ||
| More Info.: | Get all data from this article, Solution Info, Assay Method | ||
| Coagulation factor XI | |||
| Name: | Coagulation factor XI | ||
| Synonyms: | Coagulation factor XIa | Coagulation factor XIa heavy chain | Coagulation factor XIa light chain | F11 | FA11_HUMAN | FXI | Factor XIa | Factor XIa (fXIa) | PTA | Plasma thromboplastin antecedent | ||
| Type: | Enzyme | ||
| Mol. Mass.: | 70130.58 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P03951 | ||
| Residue: | 625 | ||
| Sequence: |
| ||
| BDBM12975 | |||
| BDBM12947 | |||
| Name | BDBM12975 | ||
| Synonyms: | (2S)-2-[(2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carbamoyl}amino)-3-(1H-indol-3-yl)propanamido]-N-[(2S)-5-carbamimidamido-1-oxo-1-(1,3-thiazol-2-yl)pentan-2-yl]-3-methylbutanamide | alpha-ketothiazole analogue 35 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C34H42BrN9O4S | ||
| Mol. Mass. | 752.724 | ||
| SMILES | CC(C)[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)N[C@H](C)c1ccc(Br)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)c1nccs1 |r,wU:7.19,34.36,22.24,wD:3.3,(-2.47,-.39,;-1.14,.38,;.19,-.39,;-1.14,1.92,;-2.47,2.69,;-3.81,1.92,;-3.81,.38,;-5.14,2.69,;-5.14,4.23,;-5.54,5.72,;-4.62,6.96,;-5.52,8.21,;-6.99,7.75,;-8.31,8.53,;-9.65,7.77,;-9.67,6.23,;-8.34,5.45,;-7,6.21,;-6.47,1.92,;-7.81,2.69,;-7.81,4.23,;-9.14,1.92,;-10.48,2.69,;-10.48,4.23,;-11.81,1.92,;-13.14,2.69,;-14.48,1.92,;-14.48,.38,;-15.81,-.39,;-13.14,-.39,;-11.81,.38,;.19,2.69,;.19,4.23,;1.53,1.92,;2.86,2.69,;2.86,4.23,;4.19,5,;4.19,6.54,;2.86,7.31,;2.86,8.85,;4.19,9.62,;1.53,9.62,;4.19,1.92,;4.19,.38,;5.53,2.69,;7.07,2.69,;7.54,4.16,;6.3,5.06,;5.05,4.16,)| | ||
| Structure | 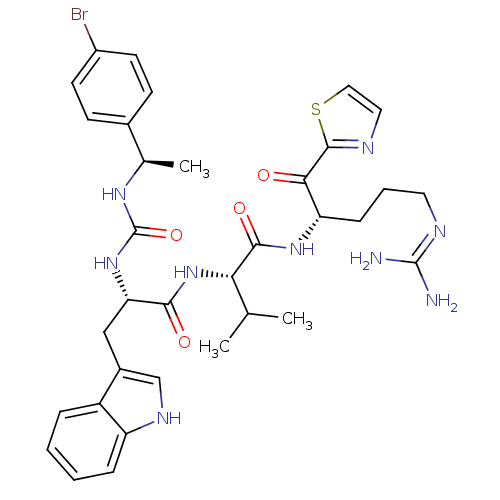
| ||