| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Serine protease 1 |
|---|
| Ligand | BDBM14127 |
|---|
| Substrate/Competitor | BDBM14141 |
|---|
| Meas. Tech. | Enzyme Inhibition Assay |
|---|
| Ki | 0.3±n/a nM |
|---|
| Citation |  Costanzo, MJ; Almond, HR; Hecker, LR; Schott, MR; Yabut, SC; Zhang, HC; Andrade-Gordon, P; Corcoran, TW; Giardino, EC; Kauffman, JA; Lewis, JM; de Garavilla, L; Haertlein, BJ; Maryanoff, BE In-depth study of tripeptide-based alpha-ketoheterocycles as inhibitors of thrombin. Effective utilization of the S1' subsite and its implications to structure-based drug design. J Med Chem48:1984-2008 (2005) [PubMed] Article Costanzo, MJ; Almond, HR; Hecker, LR; Schott, MR; Yabut, SC; Zhang, HC; Andrade-Gordon, P; Corcoran, TW; Giardino, EC; Kauffman, JA; Lewis, JM; de Garavilla, L; Haertlein, BJ; Maryanoff, BE In-depth study of tripeptide-based alpha-ketoheterocycles as inhibitors of thrombin. Effective utilization of the S1' subsite and its implications to structure-based drug design. J Med Chem48:1984-2008 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Serine protease 1 |
|---|
| Name: | Serine protease 1 |
|---|
| Synonyms: | Beta-Trypsin | Cationic trypsin | PRSS1 | TRP1 | TRY1 | TRY1_BOVIN | TRYP1 | Trypsin | Trypsin I |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 25790.52 |
|---|
| Organism: | Bos taurus (bovine) |
|---|
| Description: | P00760 |
|---|
| Residue: | 246 |
|---|
| Sequence: | MKTFIFLALLGAAVAFPVDDDDKIVGGYTCGANTVPYQVSLNSGYHFCGGSLINSQWVVS
AAHCYKSGIQVRLGEDNINVVEGNEQFISASKSIVHPSYNSNTLNNDIMLIKLKSAASLN
SRVASISLPTSCASAGTQCLISGWGNTKSSGTSYPDVLKCLKAPILSDSSCKSAYPGQIT
SNMFCAGYLEGGKDSCQGDSGGPVVCSGKLQGIVSWGSGCAQKNKPGVYTKVCNYVSWIK
QTIASN
|
|
|
|---|
| BDBM14127 |
|---|
| BDBM14141 |
|---|
| Name | BDBM14127 |
|---|
| Synonyms: | 2-(5-carbamimidamido-2-{[(2S)-1-[(2R)-2-(methylamino)-3-phenylpropanoyl]pyrrolidin-2-yl]formamido}pentanoyl)-1,3-benzothiazole-6-carboxamide | 2-ketobenzothiazole 67 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H36N8O4S |
|---|
| Mol. Mass. | 592.712 |
|---|
| SMILES | [#6]-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-c1nc2ccc(cc2s1)-[#6](-[#7])=O |r| |
|---|
| Structure | 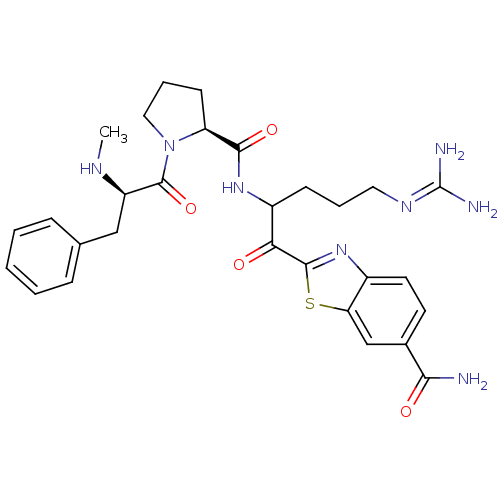
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Costanzo, MJ; Almond, HR; Hecker, LR; Schott, MR; Yabut, SC; Zhang, HC; Andrade-Gordon, P; Corcoran, TW; Giardino, EC; Kauffman, JA; Lewis, JM; de Garavilla, L; Haertlein, BJ; Maryanoff, BE In-depth study of tripeptide-based alpha-ketoheterocycles as inhibitors of thrombin. Effective utilization of the S1' subsite and its implications to structure-based drug design. J Med Chem48:1984-2008 (2005) [PubMed] Article
Costanzo, MJ; Almond, HR; Hecker, LR; Schott, MR; Yabut, SC; Zhang, HC; Andrade-Gordon, P; Corcoran, TW; Giardino, EC; Kauffman, JA; Lewis, JM; de Garavilla, L; Haertlein, BJ; Maryanoff, BE In-depth study of tripeptide-based alpha-ketoheterocycles as inhibitors of thrombin. Effective utilization of the S1' subsite and its implications to structure-based drug design. J Med Chem48:1984-2008 (2005) [PubMed] Article