| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Lysosomal acid glucosylceramidase |
|---|
| Ligand | BDBM18439 |
|---|
| Substrate/Competitor | BDBM18429 |
|---|
| Meas. Tech. | GC Enzyme Assay |
|---|
| pH | 5.9±n/a |
|---|
| Temperature | 294.15±n/a K |
|---|
| Ki | 102±n/a nM |
|---|
| IC50 | 168±n/a nM |
|---|
| Km | 28000±n/a nM |
|---|
| Citation |  Zheng, W; Padia, J; Urban, DJ; Jadhav, A; Goker-Alpan, O; Simeonov, A; Goldin, E; Auld, D; LaMarca, ME; Inglese, J; Austin, CP; Sidransky, E Three classes of glucocerebrosidase inhibitors identified by quantitative high-throughput screening are chaperone leads for Gaucher disease. Proc Natl Acad Sci U S A104:13192-7 (2007) [PubMed] Article Zheng, W; Padia, J; Urban, DJ; Jadhav, A; Goker-Alpan, O; Simeonov, A; Goldin, E; Auld, D; LaMarca, ME; Inglese, J; Austin, CP; Sidransky, E Three classes of glucocerebrosidase inhibitors identified by quantitative high-throughput screening are chaperone leads for Gaucher disease. Proc Natl Acad Sci U S A104:13192-7 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Inhibition_Run data, Solution Info, Assay Method |
|---|
| |
| Lysosomal acid glucosylceramidase |
|---|
| Name: | Lysosomal acid glucosylceramidase |
|---|
| Synonyms: | Acid beta-glucosidase | Alglucerase | Beta-glucocerebrosidase | Beta-glucocerebrosidase (GC) | D-glucosyl-N-acylsphingosine glucohydrolase | GBA | GBA1 | GBA1_HUMAN | GC | GCase | GLUC | Glucocerebrosidase (GBA) | Glucosylceramidase (GBA) | Glucosylceramidase (GCase) | Glucosylceramidase precursor (Beta-glucocerebrosidase) (Acid beta-glucosidase) (D-glucosyl-N-acylsphingosine glucohydrolase) (Alglucerase) (Imiglucerase) | Imiglucerase | beta-glucocerebrosidase (GCase) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 59724.64 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | The beta-Glu activity was measured with commercially available beta-glucocerebrosidase (Ceredase) as the enzyme source. |
|---|
| Residue: | 536 |
|---|
| Sequence: | MEFSSPSREECPKPLSRVSIMAGSLTGLLLLQAVSWASGARPCIPKSFGYSSVVCVCNAT
YCDSFDPPTFPALGTFSRYESTRSGRRMELSMGPIQANHTGTGLLLTLQPEQKFQKVKGF
GGAMTDAAALNILALSPPAQNLLLKSYFSEEGIGYNIIRVPMASCDFSIRTYTYADTPDD
FQLHNFSLPEEDTKLKIPLIHRALQLAQRPVSLLASPWTSPTWLKTNGAVNGKGSLKGQP
GDIYHQTWARYFVKFLDAYAEHKLQFWAVTAENEPSAGLLSGYPFQCLGFTPEHQRDFIA
RDLGPTLANSTHHNVRLLMLDDQRLLLPHWAKVVLTDPEAAKYVHGIAVHWYLDFLAPAK
ATLGETHRLFPNTMLFASEACVGSKFWEQSVRLGSWDRGMQYSHSIITNLLYHVVGWTDW
NLALNPEGGPNWVRNFVDSPIIVDITKDTFYKQPMFYHLGHFSKFIPEGSQRVGLVASQK
NDLDAVALMHPDGSAVVVVLNRSSKDVPLTIKDPAVGFLETISPGYSIHTYLWRRQ
|
|
|
|---|
| BDBM18439 |
|---|
| BDBM18429 |
|---|
| Name | BDBM18439 |
|---|
| Synonyms: | N-(5-methyl-1,2-oxazol-3-yl)-4-[(4-methylbenzene)sulfonamido]benzamide | NCGC00058635 | Sulfonamide analogue, 10 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H17N3O4S |
|---|
| Mol. Mass. | 371.41 |
|---|
| SMILES | Cc1cc(NC(=O)c2ccc(NS(=O)(=O)c3ccc(C)cc3)cc2)no1 |
|---|
| Structure | 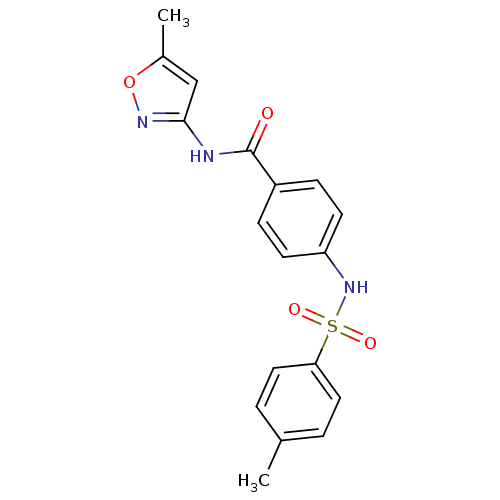
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zheng, W; Padia, J; Urban, DJ; Jadhav, A; Goker-Alpan, O; Simeonov, A; Goldin, E; Auld, D; LaMarca, ME; Inglese, J; Austin, CP; Sidransky, E Three classes of glucocerebrosidase inhibitors identified by quantitative high-throughput screening are chaperone leads for Gaucher disease. Proc Natl Acad Sci U S A104:13192-7 (2007) [PubMed] Article
Zheng, W; Padia, J; Urban, DJ; Jadhav, A; Goker-Alpan, O; Simeonov, A; Goldin, E; Auld, D; LaMarca, ME; Inglese, J; Austin, CP; Sidransky, E Three classes of glucocerebrosidase inhibitors identified by quantitative high-throughput screening are chaperone leads for Gaucher disease. Proc Natl Acad Sci U S A104:13192-7 (2007) [PubMed] Article