| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cathepsin S |
|---|
| Ligand | BDBM19634 |
|---|
| Substrate/Competitor | BDBM19546 |
|---|
| Meas. Tech. | Enzyme Inhibition Assay |
|---|
| pH | 5.5±n/a |
|---|
| Temperature | 310.15±n/a K |
|---|
| Ki | 12±n/a nM |
|---|
| Citation |  Tully, DC; Liu, H; Alper, PB; Chatterjee, AK; Epple, R; Roberts, MJ; Williams, JA; Nguyen, KT; Woodmansee, DH; Tumanut, C; Li, J; Spraggon, G; Chang, J; Tuntland, T; Harris, JL; Karanewsky, DS Synthesis and evaluation of arylaminoethyl amides as noncovalent inhibitors of cathepsin S. Part 3: heterocyclic P3. Bioorg Med Chem Lett16:1975-80 (2006) [PubMed] Article Tully, DC; Liu, H; Alper, PB; Chatterjee, AK; Epple, R; Roberts, MJ; Williams, JA; Nguyen, KT; Woodmansee, DH; Tumanut, C; Li, J; Spraggon, G; Chang, J; Tuntland, T; Harris, JL; Karanewsky, DS Synthesis and evaluation of arylaminoethyl amides as noncovalent inhibitors of cathepsin S. Part 3: heterocyclic P3. Bioorg Med Chem Lett16:1975-80 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Inhibition_Run data, Solution Info, Assay Method |
|---|
| |
| Cathepsin S |
|---|
| Name: | Cathepsin S |
|---|
| Synonyms: | CATS_HUMAN | CTSS | Cathepsin S (Cat S) | cathepsin S preproprotein |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 37507.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P25774 |
|---|
| Residue: | 331 |
|---|
| Sequence: | MKRLVCVLLVCSSAVAQLHKDPTLDHHWHLWKKTYGKQYKEKNEEAVRRLIWEKNLKFVM
LHNLEHSMGMHSYDLGMNHLGDMTSEEVMSLMSSLRVPSQWQRNITYKSNPNRILPDSVD
WREKGCVTEVKYQGSCGACWAFSAVGALEAQLKLKTGKLVSLSAQNLVDCSTEKYGNKGC
NGGFMTTAFQYIIDNKGIDSDASYPYKAMDQKCQYDSKYRAATCSKYTELPYGREDVLKE
AVANKGPVSVGVDARHPSFFLYRSGVYYEPSCTQNVNHGVLVVGYGDLNGKEYWLVKNSW
GHNFGEEGYIRMARNKGNHCGIASFPSYPEI
|
|
|
|---|
| BDBM19634 |
|---|
| BDBM19546 |
|---|
| Name | BDBM19634 |
|---|
| Synonyms: | (3S)-3-[(2S)-2-(1,3-benzoxazol-2-ylamino)-3-cyclohexylpropanamido]-4-(5-fluoro-2,3-dihydro-1H-indol-1-yl)butanoic acid | Heterocyclic arylaminoethyl amide, 13f |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H33FN4O4 |
|---|
| Mol. Mass. | 508.5844 |
|---|
| SMILES | OC(=O)C[C@@H](CN1CCc2cc(F)ccc12)NC(=O)[C@H](CC1CCCCC1)Nc1nc2ccccc2o1 |r| |
|---|
| Structure | 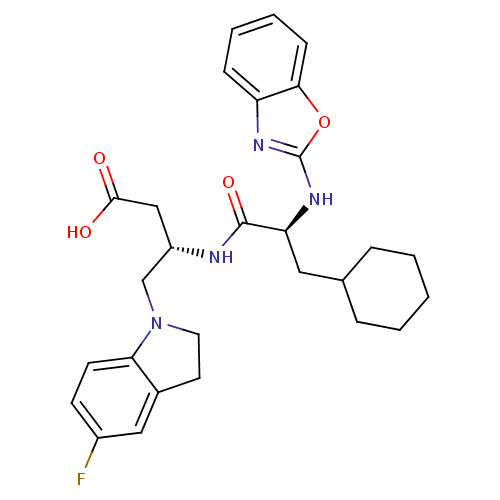
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tully, DC; Liu, H; Alper, PB; Chatterjee, AK; Epple, R; Roberts, MJ; Williams, JA; Nguyen, KT; Woodmansee, DH; Tumanut, C; Li, J; Spraggon, G; Chang, J; Tuntland, T; Harris, JL; Karanewsky, DS Synthesis and evaluation of arylaminoethyl amides as noncovalent inhibitors of cathepsin S. Part 3: heterocyclic P3. Bioorg Med Chem Lett16:1975-80 (2006) [PubMed] Article
Tully, DC; Liu, H; Alper, PB; Chatterjee, AK; Epple, R; Roberts, MJ; Williams, JA; Nguyen, KT; Woodmansee, DH; Tumanut, C; Li, J; Spraggon, G; Chang, J; Tuntland, T; Harris, JL; Karanewsky, DS Synthesis and evaluation of arylaminoethyl amides as noncovalent inhibitors of cathepsin S. Part 3: heterocyclic P3. Bioorg Med Chem Lett16:1975-80 (2006) [PubMed] Article