| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tyrosine-protein kinase JAK3 |
|---|
| Ligand | BDBM14949 |
|---|
| Substrate/Competitor | BDBM17296 |
|---|
| Meas. Tech. | HTRF Kinase Inhibition Assay |
|---|
| IC50 | 20±n/a nM |
|---|
| Citation |  DiMauro, EF; Newcomb, J; Nunes, JJ; Bemis, JE; Boucher, C; Chai, L; Chaffee, SC; Deak, HL; Epstein, LF; Faust, T; Gallant, P; Gore, A; Gu, Y; Henkle, B; Hsieh, F; Huang, X; Kim, JL; Lee, JH; Martin, MW; McGowan, DC; Metz, D; Mohn, D; Morgenstern, KA; Oliveira-dos-Santos, A; Patel, VF; Powers, D; Rose, PE; Schneider, S; Tomlinson, SA; Tudor, YY; Turci, SM; Welcher, AA; Zhao, H; Zhu, L; Zhu, X Structure-guided design of aminopyrimidine amides as potent, selective inhibitors of lymphocyte specific kinase: synthesis, structure-activity relationships, and inhibition of in vivo T cell activation. J Med Chem51:1681-94 (2008) [PubMed] Article DiMauro, EF; Newcomb, J; Nunes, JJ; Bemis, JE; Boucher, C; Chai, L; Chaffee, SC; Deak, HL; Epstein, LF; Faust, T; Gallant, P; Gore, A; Gu, Y; Henkle, B; Hsieh, F; Huang, X; Kim, JL; Lee, JH; Martin, MW; McGowan, DC; Metz, D; Mohn, D; Morgenstern, KA; Oliveira-dos-Santos, A; Patel, VF; Powers, D; Rose, PE; Schneider, S; Tomlinson, SA; Tudor, YY; Turci, SM; Welcher, AA; Zhao, H; Zhu, L; Zhu, X Structure-guided design of aminopyrimidine amides as potent, selective inhibitors of lymphocyte specific kinase: synthesis, structure-activity relationships, and inhibition of in vivo T cell activation. J Med Chem51:1681-94 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Tyrosine-protein kinase JAK3 |
|---|
| Name: | Tyrosine-protein kinase JAK3 |
|---|
| Synonyms: | JAK-3 | JAK3 | JAK3_HUMAN | Janus kinase 3 | Janus kinase 3 (JAK3) | Janus kinase 3 JAK3 | L-JAK | Leukocyte janus kinase |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 125111.08 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P52333 |
|---|
| Residue: | 1124 |
|---|
| Sequence: | MAPPSEETPLIPQRSCSLLSTEAGALHVLLPARGPGPPQRLSFSFGDHLAEDLCVQAAKA
SGILPVYHSLFALATEDLSCWFPPSHIFSVEDASTQVLLYRIRFYFPNWFGLEKCHRFGL
RKDLASAILDLPVLEHLFAQHRSDLVSGRLPVGLSLKEQGECLSLAVLDLARMAREQAQR
PGELLKTVSYKACLPPSLRDLIQGLSFVTRRRIRRTVRRALRRVAACQADRHSLMAKYIM
DLERLDPAGAAETFHVGLPGALGGHDGLGLLRVAGDGGIAWTQGEQEVLQPFCDFPEIVD
ISIKQAPRVGPAGEHRLVTVTRTDNQILEAEFPGLPEALSFVALVDGYFRLTTDSQHFFC
KEVAPPRLLEEVAEQCHGPITLDFAINKLKTGGSRPGSYVLRRSPQDFDSFLLTVCVQNP
LGPDYKGCLIRRSPTGTFLLVGLSRPHSSLRELLATCWDGGLHVDGVAVTLTSCCIPRPK
EKSNLIVVQRGHSPPTSSLVQPQSQYQLSQMTFHKIPADSLEWHENLGHGSFTKIYRGCR
HEVVDGEARKTEVLLKVMDAKHKNCMESFLEAASLMSQVSYRHLVLLHGVCMAGDSTMVQ
EFVHLGAIDMYLRKRGHLVPASWKLQVVKQLAYALNYLEDKGLPHGNVSARKVLLAREGA
DGSPPFIKLSDPGVSPAVLSLEMLTDRIPWVAPECLREAQTLSLEADKWGFGATVWEVFS
GVTMPISALDPAKKLQFYEDRQQLPAPKWTELALLIQQCMAYEPVQRPSFRAVIRDLNSL
ISSDYELLSDPTPGALAPRDGLWNGAQLYACQDPTIFEERHLKYISQLGKGNFGSVELCR
YDPLGDNTGALVAVKQLQHSGPDQQRDFQREIQILKALHSDFIVKYRGVSYGPGRQSLRL
VMEYLPSGCLRDFLQRHRARLDASRLLLYSSQICKGMEYLGSRRCVHRDLAARNILVESE
AHVKIADFGLAKLLPLDKDYYVVREPGQSPIFWYAPESLSDNIFSRQSDVWSFGVVLYEL
FTYCDKSCSPSAEFLRMMGCERDVPALCRLLELLEEGQRLPAPPACPAEVHELMKLCWAP
SPQDRPSFSALGPQLDMLWSGSRGCETHAFTAHPEGKHHSLSFS
|
|
|
|---|
| BDBM14949 |
|---|
| BDBM17296 |
|---|
| Name | BDBM14949 |
|---|
| Synonyms: | 2-aminoquinazoline 5 | 3-(2-aminoquinazolin-6-yl)-4-methyl-N-[3-(trifluoromethyl)phenyl]benzamide | CHEMBL385937 | JMC511681 Compound 34 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H17F3N4O |
|---|
| Mol. Mass. | 422.4025 |
|---|
| SMILES | Cc1ccc(cc1-c1ccc2nc(N)ncc2c1)C(=O)Nc1cccc(c1)C(F)(F)F |
|---|
| Structure | 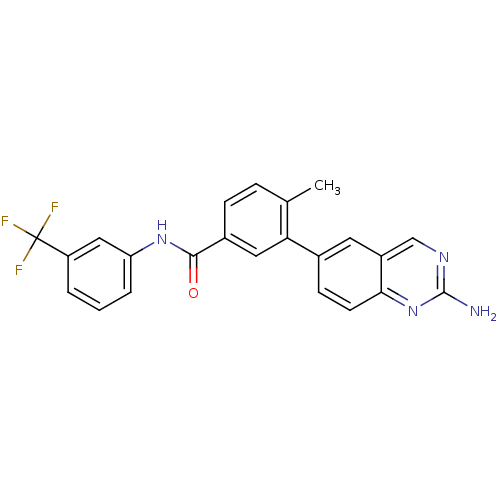
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 DiMauro, EF; Newcomb, J; Nunes, JJ; Bemis, JE; Boucher, C; Chai, L; Chaffee, SC; Deak, HL; Epstein, LF; Faust, T; Gallant, P; Gore, A; Gu, Y; Henkle, B; Hsieh, F; Huang, X; Kim, JL; Lee, JH; Martin, MW; McGowan, DC; Metz, D; Mohn, D; Morgenstern, KA; Oliveira-dos-Santos, A; Patel, VF; Powers, D; Rose, PE; Schneider, S; Tomlinson, SA; Tudor, YY; Turci, SM; Welcher, AA; Zhao, H; Zhu, L; Zhu, X Structure-guided design of aminopyrimidine amides as potent, selective inhibitors of lymphocyte specific kinase: synthesis, structure-activity relationships, and inhibition of in vivo T cell activation. J Med Chem51:1681-94 (2008) [PubMed] Article
DiMauro, EF; Newcomb, J; Nunes, JJ; Bemis, JE; Boucher, C; Chai, L; Chaffee, SC; Deak, HL; Epstein, LF; Faust, T; Gallant, P; Gore, A; Gu, Y; Henkle, B; Hsieh, F; Huang, X; Kim, JL; Lee, JH; Martin, MW; McGowan, DC; Metz, D; Mohn, D; Morgenstern, KA; Oliveira-dos-Santos, A; Patel, VF; Powers, D; Rose, PE; Schneider, S; Tomlinson, SA; Tudor, YY; Turci, SM; Welcher, AA; Zhao, H; Zhu, L; Zhu, X Structure-guided design of aminopyrimidine amides as potent, selective inhibitors of lymphocyte specific kinase: synthesis, structure-activity relationships, and inhibition of in vivo T cell activation. J Med Chem51:1681-94 (2008) [PubMed] Article