| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glycogen phosphorylase, liver form |
|---|
| Ligand | BDBM27744 |
|---|
| Substrate/Competitor | BDBM24362 |
|---|
| Meas. Tech. | Human Liver GPa Enzymatic Activity Assay |
|---|
| pH | 7.6±n/a |
|---|
| Temperature | 298.15±n/a K |
|---|
| IC50 | 7±10 nM |
|---|
| Citation |  Thomson, SA; Banker, P; Bickett, DM; Boucheron, JA; Carter, HL; Clancy, DC; Cooper, JP; Dickerson, SH; Garrido, DM; Nolte, RT; Peat, AJ; Sheckler, LR; Sparks, SM; Tavares, FX; Wang, L; Wang, TY; Weiel, JE Anthranilimide based glycogen phosphorylase inhibitors for the treatment of type 2 diabetes. Part 3: X-ray crystallographic characterization, core and urea optimization and in vivo efficacy. Bioorg Med Chem Lett19:1177-82 (2009) [PubMed] Article Thomson, SA; Banker, P; Bickett, DM; Boucheron, JA; Carter, HL; Clancy, DC; Cooper, JP; Dickerson, SH; Garrido, DM; Nolte, RT; Peat, AJ; Sheckler, LR; Sparks, SM; Tavares, FX; Wang, L; Wang, TY; Weiel, JE Anthranilimide based glycogen phosphorylase inhibitors for the treatment of type 2 diabetes. Part 3: X-ray crystallographic characterization, core and urea optimization and in vivo efficacy. Bioorg Med Chem Lett19:1177-82 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Inhibition_Run data, Solution Info, Assay Method |
|---|
| |
| Glycogen phosphorylase, liver form |
|---|
| Name: | Glycogen phosphorylase, liver form |
|---|
| Synonyms: | Glycogen Phosphorylase (PYGL) | Glycogen Phosphorylase, liver form | Liver glycogen phosphorylase | PYGL | PYGL_HUMAN |
|---|
| Type: | Homodimer |
|---|
| Mol. Mass.: | 97153.98 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Dimers associate into a tetramer to form the enzymatically active phosphorylase A. |
|---|
| Residue: | 847 |
|---|
| Sequence: | MAKPLTDQEKRRQISIRGIVGVENVAELKKSFNRHLHFTLVKDRNVATTRDYYFALAHTV
RDHLVGRWIRTQQHYYDKCPKRVYYLSLEFYMGRTLQNTMINLGLQNACDEAIYQLGLDI
EELEEIEEDAGLGNGGLGRLAACFLDSMATLGLAAYGYGIRYEYGIFNQKIRDGWQVEEA
DDWLRYGNPWEKSRPEFMLPVHFYGKVEHTNTGTKWIDTQVVLALPYDTPVPGYMNNTVN
TMRLWSARAPNDFNLRDFNVGDYIQAVLDRNLAENISRVLYPNDNFFEGKELRLKQEYFV
VAATLQDIIRRFKASKFGSTRGAGTVFDAFPDQVAIQLNDTHPALAIPELMRIFVDIEKL
PWSKAWELTQKTFAYTNHTVLPEALERWPVDLVEKLLPRHLEIIYEINQKHLDRIVALFP
KDVDRLRRMSLIEEEGSKRINMAHLCIVGSHAVNGVAKIHSDIVKTKVFKDFSELEPDKF
QNKTNGITPRRWLLLCNPGLAELIAEKIGEDYVKDLSQLTKLHSFLGDDVFLRELAKVKQ
ENKLKFSQFLETEYKVKINPSSMFDVQVKRIHEYKRQLLNCLHVITMYNRIKKDPKKLFV
PRTVIIGGKAAPGYHMAKMIIKLITSVADVVNNDPMVGSKLKVIFLENYRVSLAEKVIPA
TDLSEQISTAGTEASGTGNMKFMLNGALTIGTMDGANVEMAEEAGEENLFIFGMRIDDVA
ALDKKGYEAKEYYEALPELKLVIDQIDNGFFSPKQPDLFKDIINMLFYHDRFKVFADYEA
YVKCQDKVSQLYMNPKAWNTMVLKNIAASGKFSSDRTIKEYAQNIWNVEPSDLKISLSNE
SNKVNGN
|
|
|
|---|
| BDBM27744 |
|---|
| BDBM24362 |
|---|
| Name | BDBM27744 |
|---|
| Synonyms: | (2S,3R)-3-(tert-butoxy)-2-{[4-(3,4-difluorophenyl)-2-{[(2,6-dimethyl-4-propylphenyl)carbamoyl]amino}phenyl]formamido}butanoic acid | anthranilimide based compound, 20 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C33H39F2N3O5 |
|---|
| Mol. Mass. | 595.6767 |
|---|
| SMILES | CCCc1cc(C)c(NC(=O)Nc2cc(ccc2C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)-c2ccc(F)c(F)c2)c(C)c1 |r| |
|---|
| Structure | 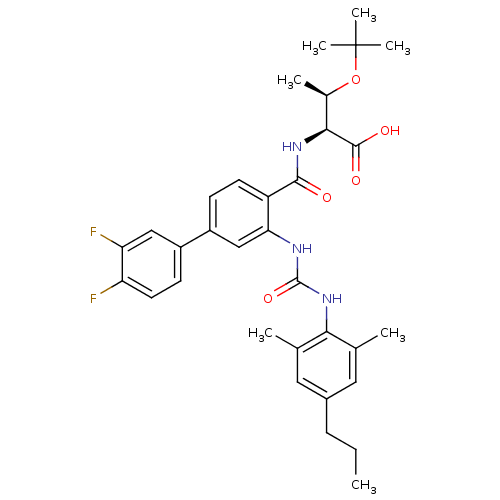
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Thomson, SA; Banker, P; Bickett, DM; Boucheron, JA; Carter, HL; Clancy, DC; Cooper, JP; Dickerson, SH; Garrido, DM; Nolte, RT; Peat, AJ; Sheckler, LR; Sparks, SM; Tavares, FX; Wang, L; Wang, TY; Weiel, JE Anthranilimide based glycogen phosphorylase inhibitors for the treatment of type 2 diabetes. Part 3: X-ray crystallographic characterization, core and urea optimization and in vivo efficacy. Bioorg Med Chem Lett19:1177-82 (2009) [PubMed] Article
Thomson, SA; Banker, P; Bickett, DM; Boucheron, JA; Carter, HL; Clancy, DC; Cooper, JP; Dickerson, SH; Garrido, DM; Nolte, RT; Peat, AJ; Sheckler, LR; Sparks, SM; Tavares, FX; Wang, L; Wang, TY; Weiel, JE Anthranilimide based glycogen phosphorylase inhibitors for the treatment of type 2 diabetes. Part 3: X-ray crystallographic characterization, core and urea optimization and in vivo efficacy. Bioorg Med Chem Lett19:1177-82 (2009) [PubMed] Article