| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Eukaryotic translation initiation factor 4 gamma 1 |
|---|
| Ligand | BDBM48803 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | Dose Response Confirmation for Small Molecule Inhibitors of Eukaryotic Translation Initiation |
|---|
| IC50 | 15100±n/a nM |
|---|
| Citation |  PubChem, PC Dose Response Confirmation for Small Molecule Inhibitors of Eukaryotic Translation Initiation PubChem Bioassay(2009)[AID] PubChem, PC Dose Response Confirmation for Small Molecule Inhibitors of Eukaryotic Translation Initiation PubChem Bioassay(2009)[AID] |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Eukaryotic translation initiation factor 4 gamma 1 |
|---|
| Name: | Eukaryotic translation initiation factor 4 gamma 1 |
|---|
| Synonyms: | EIF4F | EIF4G | EIF4G1 | EIF4GI | IF4G1_HUMAN | eukaryotic translation initiation factor 4 gamma, 1 isoform 4 |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 175455.17 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q04637 |
|---|
| Residue: | 1599 |
|---|
| Sequence: | MNKAPQSTGPPPAPSPGLPQPAFPPGQTAPVVFSTPQATQMNTPSQPRQHFYPSRAQPPS
SAASRVQSAAPARPGPAAHVYPAGSQVMMIPSQISYPASQGAYYIPGQGRSTYVVPTQQY
PVQPGAPGFYPGASPTEFGTYAGAYYPAQGVQQFPTGVAPTPVLMNQPPQIAPKRERKTI
RIRDPNQGGKDITEEIMSGARTASTPTPPQTGGGLEPQANGETPQVAVIVRPDDRSQGAI
IADRPGLPGPEHSPSESQPSSPSPTPSPSPVLEPGSEPNLAVLSIPGDTMTTIQMSVEES
TPISRETGEPYRLSPEPTPLAEPILEVEVTLSKPVPESEFSSSPLQAPTPLASHTVEIHE
PNGMVPSEDLEPEVESSPELAPPPACPSESPVPIAPTAQPEELLNGAPSPPAVDLSPVSE
PEEQAKEVTASMAPPTIPSATPATAPSATSPAQEEEMEEEEEEEEGEAGEAGEAESEKGG
EELLPPESTPIPANLSQNLEAAAATQVAVSVPKRRRKIKELNKKEAVGDLLDAFKEANPA
VPEVENQPPAGSNPGPESEGSGVPPRPEEADETWDSKEDKIHNAENIQPGEQKYEYKSDQ
WKPLNLEEKKRYDREFLLGFQFIFASMQKPEGLPHISDVVLDKANKTPLRPLDPTRLQGI
NCGPDFTPSFANLGRTTLSTRGPPRGGPGGELPRGPAGLGPRRSQQGPRKEPRKIIATVL
MTEDIKLNKAEKAWKPSSKRTAADKDRGEEDADGSKTQDLFRRVRSILNKLTPQMFQQLM
KQVTQLAIDTEERLKGVIDLIFEKAISEPNFSVAYANMCRCLMALKVPTTEKPTVTVNFR
KLLLNRCQKEFEKDKDDDEVFEKKQKEMDEAATAEERGRLKEELEEARDIARRRSLGNIK
FIGELFKLKMLTEAIMHDCVVKLLKNHDEESLECLCRLLTTIGKDLDFEKAKPRMDQYFN
QMEKIIKEKKTSSRIRFMLQDVLDLRGSNWVPRRGDQGPKTIDQIHKEAEMEEHREHIKV
QQLMAKGSDKRRGGPPGPPISRGLPLVDDGGWNTVPISKGSRPIDTSRLTKITKPGSIDS
NNQLFAPGGRLSWGKGSSGGSGAKPSDAASEAARPATSTLNRFSALQQAVPTESTDNRRV
VQRSSLSRERGEKAGDRGDRLERSERGGDRGDRLDRARTPATKRSFSKEVEERSRERPSQ
PEGLRKAASLTEDRDRGRDAVKREAALPPVSPLKAALSEEELEKKSKAIIEEYLHLNDMK
EAVQCVQELASPSLLFIFVRHGVESTLERSAIAREHMGQLLHQLLCAGHLSTAQYYQGLY
EILELAEDMEIDIPHVWLYLAELVTPILQEGGVPMGELFREITKPLRPLGKAASLLLEIL
GLLCKSMGPKKVGTLWREAGLSWKEFLPEGQDIGAFVAEQKVEYTLGEESEAPGQRALPS
EELNRQLEKLLKEGSSNQRVFDWIEANLSEQQIVSNTLVRALMTAVCYSAIIFETPLRVD
VAVLKARAKLLQKYLCDEQKELQALYALQALVVTLEQPPNLLRMFFDALYDEDVVKEDAF
YSWESSKDPAEQQGKGVALKSVTAFFKWLREAEEESDHN
|
|
|
|---|
| BDBM48803 |
|---|
| n/a |
|---|
| Name | BDBM48803 |
|---|
| Synonyms: | 2-chloranyl-5-[5-[(E)-[4-oxidanylidene-3-(phenylmethyl)-2-sulfanylidene-1,3-thiazolidin-5-ylidene]methyl]furan-2-yl]benzoic acid | 2-chloro-5-[5-[(E)-[4-oxo-3-(phenylmethyl)-2-sulfanylidene-5-thiazolidinylidene]methyl]-2-furanyl]benzoic acid | 5-[5-[(E)-(3-benzyl-4-keto-2-thioxo-thiazolidin-5-ylidene)methyl]-2-furyl]-2-chloro-benzoic acid | 5-[5-[(E)-(3-benzyl-4-oxo-2-sulfanylidene-1,3-thiazolidin-5-ylidene)methyl]furan-2-yl]-2-chlorobenzoic acid | 5-{5-[(3-benzyl-4-oxo-2-thioxo-1,3-thiazolidin-5-ylidene)methyl]-2-furyl}-2-chlorobenzoic acid | MLS000690029 | SMR000298763 | cid_2287238 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H14ClNO4S2 |
|---|
| Mol. Mass. | 455.934 |
|---|
| SMILES | OC(=O)c1cc(ccc1Cl)-c1ccc(\C=C2\SC(=S)N(Cc3ccccc3)C2=O)o1 |
|---|
| Structure | 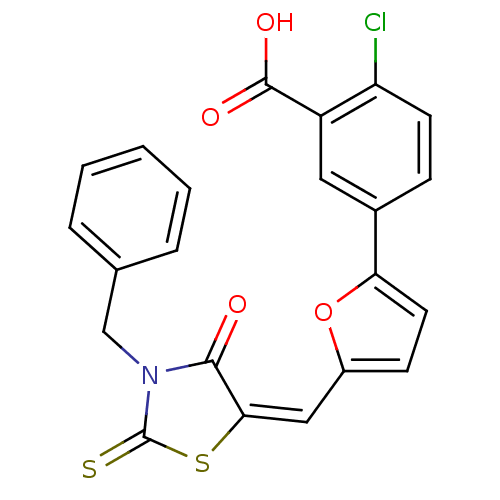
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 PubChem, PC Dose Response Confirmation for Small Molecule Inhibitors of Eukaryotic Translation Initiation PubChem Bioassay(2009)[AID]
PubChem, PC Dose Response Confirmation for Small Molecule Inhibitors of Eukaryotic Translation Initiation PubChem Bioassay(2009)[AID]