

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Cholecystokinin | ||
| Ligand | BDBM50040671 | ||
| Substrate/Competitor | n/a | ||
| Ki | 0.1±n/a nM | ||
| Comments | PDSP_2244 | ||
| Citation |  Patel, S; Smith, AJ; Chapman, KL; Fletcher, AE; Kemp, JA; Marshall, GR; Hargreaves, RJ; Ryecroft, W; Iversen, LL; Iversen, SD Biological properties of the benzodiazepine amidine derivative L-740,093, a cholecystokinin-B/gastrin receptor antagonist with high affinity in vitro and high potency in vivo. Mol Pharmacol46:943-8 (1994) [PubMed] Patel, S; Smith, AJ; Chapman, KL; Fletcher, AE; Kemp, JA; Marshall, GR; Hargreaves, RJ; Ryecroft, W; Iversen, LL; Iversen, SD Biological properties of the benzodiazepine amidine derivative L-740,093, a cholecystokinin-B/gastrin receptor antagonist with high affinity in vitro and high potency in vivo. Mol Pharmacol46:943-8 (1994) [PubMed] | ||
| More Info.: | Get all data from this article | ||
| Cholecystokinin | |||
| Name: | Cholecystokinin | ||
| Synonyms: | CCK | CCKN_PIG | Cholecystokinin B | ||
| Type: | Enzyme Catalytic Domain | ||
| Mol. Mass.: | 12528.64 | ||
| Organism: | GUINEA PIG | ||
| Description: | Cholecystokinin B 0 GUINEA PIG::P01356 | ||
| Residue: | 114 | ||
| Sequence: |
| ||
| BDBM50040671 | |||
| n/a | |||
| Name | BDBM50040671 | ||
| Synonyms: | 1-[5-(3-Aza-bicyclo[3.2.2]non-3-yl)-1-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl]-3-m-tolyl-urea | CHEMBL349291 | L-740,093 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C26H31N5O2 | ||
| Mol. Mass. | 445.5566 | ||
| SMILES | CN1c2ccccc2C(=NC(NC(=O)Nc2cccc(C)c2)C1=O)N1CC2CCC(CC2)C1 |c:9,(7.22,-6.97,;7.67,-8.45,;6.69,-9.39,;5.36,-8.63,;4.02,-9.39,;4.02,-10.93,;5.36,-11.69,;6.71,-10.96,;7.63,-11.85,;9.14,-11.6,;9.86,-10.19,;11.4,-10.21,;12.13,-11.53,;11.35,-12.86,;13.67,-11.57,;14.48,-10.26,;13.72,-8.91,;14.5,-7.6,;16.04,-7.62,;16.81,-8.95,;18.33,-8.98,;16.01,-10.29,;9.18,-8.72,;10.1,-7.49,;7.17,-13.34,;5.55,-13.5,;4.73,-14.54,;5.14,-16.05,;6.65,-16.64,;8.09,-15.8,;7.22,-14.74,;6.1,-14.2,;8.22,-14.23,)| | ||
| Structure | 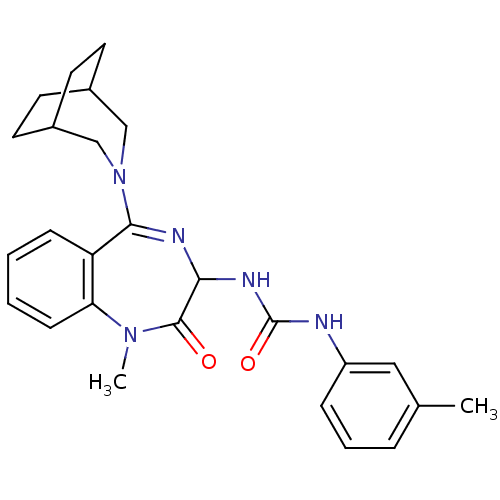
| ||