| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 3A4 |
|---|
| Ligand | BDBM50257064 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1688648 (CHEMBL4039218) |
|---|
| IC50 | >30000±n/a nM |
|---|
| Citation |  Felts, AS; Rodriguez, AL; Blobaum, AL; Morrison, RD; Bates, BS; Thompson Gray, A; Rook, JM; Tantawy, MN; Byers, FW; Chang, S; Venable, DF; Luscombe, VB; Tamagnan, GD; Niswender, CM; Daniels, JS; Jones, CK; Conn, PJ; Lindsley, CW; Emmitte, KA Discovery of N-(5-Fluoropyridin-2-yl)-6-methyl-4-(pyrimidin-5-yloxy)picolinamide (VU0424238): A Novel Negative Allosteric Modulator of Metabotropic Glutamate Receptor Subtype 5 Selected for Clinical Evaluation. J Med Chem60:5072-5085 (2017) [PubMed] Article Felts, AS; Rodriguez, AL; Blobaum, AL; Morrison, RD; Bates, BS; Thompson Gray, A; Rook, JM; Tantawy, MN; Byers, FW; Chang, S; Venable, DF; Luscombe, VB; Tamagnan, GD; Niswender, CM; Daniels, JS; Jones, CK; Conn, PJ; Lindsley, CW; Emmitte, KA Discovery of N-(5-Fluoropyridin-2-yl)-6-methyl-4-(pyrimidin-5-yloxy)picolinamide (VU0424238): A Novel Negative Allosteric Modulator of Metabotropic Glutamate Receptor Subtype 5 Selected for Clinical Evaluation. J Med Chem60:5072-5085 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 3A4 |
|---|
| Name: | Cytochrome P450 3A4 |
|---|
| Synonyms: | Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 57349.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 503 |
|---|
| Sequence: | MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMF
DMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISI
AEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYS
MDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICV
FPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSI
IFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVV
NETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFS
KKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLG
GLLQPEKPVVLKVESRDGTVSGA
|
|
|
|---|
| BDBM50257064 |
|---|
| n/a |
|---|
| Name | BDBM50257064 |
|---|
| Synonyms: | CHEMBL2386850 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H12FN5O2 |
|---|
| Mol. Mass. | 325.2972 |
|---|
| SMILES | Cc1cc(Oc2cncnc2)cc(n1)C(=O)Nc1ccc(F)cn1 |
|---|
| Structure | 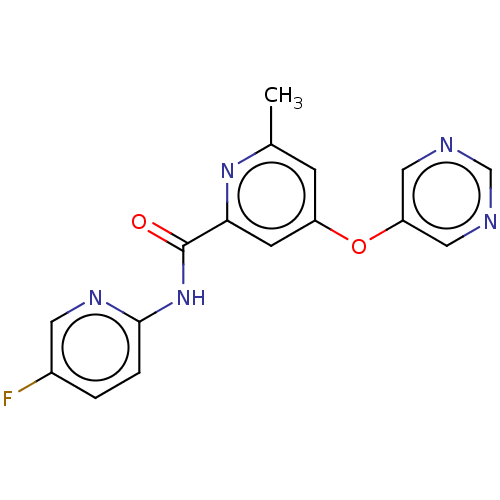
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Felts, AS; Rodriguez, AL; Blobaum, AL; Morrison, RD; Bates, BS; Thompson Gray, A; Rook, JM; Tantawy, MN; Byers, FW; Chang, S; Venable, DF; Luscombe, VB; Tamagnan, GD; Niswender, CM; Daniels, JS; Jones, CK; Conn, PJ; Lindsley, CW; Emmitte, KA Discovery of N-(5-Fluoropyridin-2-yl)-6-methyl-4-(pyrimidin-5-yloxy)picolinamide (VU0424238): A Novel Negative Allosteric Modulator of Metabotropic Glutamate Receptor Subtype 5 Selected for Clinical Evaluation. J Med Chem60:5072-5085 (2017) [PubMed] Article
Felts, AS; Rodriguez, AL; Blobaum, AL; Morrison, RD; Bates, BS; Thompson Gray, A; Rook, JM; Tantawy, MN; Byers, FW; Chang, S; Venable, DF; Luscombe, VB; Tamagnan, GD; Niswender, CM; Daniels, JS; Jones, CK; Conn, PJ; Lindsley, CW; Emmitte, KA Discovery of N-(5-Fluoropyridin-2-yl)-6-methyl-4-(pyrimidin-5-yloxy)picolinamide (VU0424238): A Novel Negative Allosteric Modulator of Metabotropic Glutamate Receptor Subtype 5 Selected for Clinical Evaluation. J Med Chem60:5072-5085 (2017) [PubMed] Article