| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cholinesterase |
|---|
| Ligand | BDBM83449 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_41570 (CHEMBL654864) |
|---|
| Ki | 520±n/a nM |
|---|
| Citation |  Kalir, A; Teomy, S; Amir, A; Fuchs, P; Lee, SA; Holsztynska, EJ; Rocki, W; Domino, EF N-allyl analogues of phencyclidine: chemical synthesis and pharmacological properties. J Med Chem27:1267-71 (1984) [PubMed] Kalir, A; Teomy, S; Amir, A; Fuchs, P; Lee, SA; Holsztynska, EJ; Rocki, W; Domino, EF N-allyl analogues of phencyclidine: chemical synthesis and pharmacological properties. J Med Chem27:1267-71 (1984) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cholinesterase |
|---|
| Name: | Cholinesterase |
|---|
| Synonyms: | Acylcholine acylhydrolase | Bche | Butyrylcholine esterase | Butyrylcholinesterase | CHLE_MOUSE | Choline esterase II | Pseudocholinesterase |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 68465.99 |
|---|
| Organism: | Mus musculus (Mouse) |
|---|
| Description: | Q03311 |
|---|
| Residue: | 603 |
|---|
| Sequence: | MQTQHTKVTQTHFLLWILLLCMPFGKSHTEEDFIITTKTGRVRGLSMPVLGGTVTAFLGI
PYAQPPLGSLRFKKPQPLNKWPDIHNATQYANSCYQNIDQAFPGFQGSEMWNPNTNLSED
CLYLNVWIPVPKPKNATVMVWIYGGGFQTGTSSLPVYDGKFLARVERVIVVSMNYRVGAL
GFLAFPGNPDAPGNMGLFDQQLALQWVQRNIAAFGGNPKSITIFGESAGAASVSLHLLCP
QSYPLFTRAILESGSSNAPWAVKHPEEARNRTLTLAKFTGCSKENEMEMIKCLRSKDPQE
ILRNERFVLPSDSILSINFGPTVDGDFLTDMPHTLLQLGKVKKAQILVGVNKDEGTAFLV
YGAPGFSKDNDSLITRKEFQEGLNMYFPGVSRLGKEAVLFYYVDWLGEQSPEVYRDALDD
VIGDYNIICPALEFTKKFAELENNAFFYFFEHRSSKLPWPEWMGVMHGYEIEFVFGLPLG
RRVNYTRAEEIFSRSIMKTWANFAKYGHPNGTQGNSTMWPVFTSTEQKYLTLNTEKSKIY
SKLRAPQCQFWRLFFPKVLEMTGDIDETEQEWKAGFHRWSNYMMDWQNQFNDYTSKKESC
TAL
|
|
|
|---|
| BDBM83449 |
|---|
| n/a |
|---|
| Name | BDBM83449 |
|---|
| Synonyms: | 1-(1-phenylcyclohexyl)piperidine;hydrochloride | MLS002320664 | PCP | PCP hydrochloride | PHENCYCLIDINE | Phencyclidine hydrochloride | SMR001338811 | cid_9795678 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H25N |
|---|
| Mol. Mass. | 243.3871 |
|---|
| SMILES | C1CCN(CC1)C1(CCCCC1)c1ccccc1 |
|---|
| Structure | 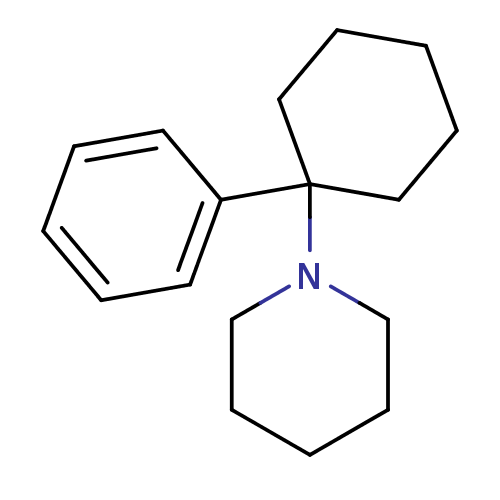
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kalir, A; Teomy, S; Amir, A; Fuchs, P; Lee, SA; Holsztynska, EJ; Rocki, W; Domino, EF N-allyl analogues of phencyclidine: chemical synthesis and pharmacological properties. J Med Chem27:1267-71 (1984) [PubMed]
Kalir, A; Teomy, S; Amir, A; Fuchs, P; Lee, SA; Holsztynska, EJ; Rocki, W; Domino, EF N-allyl analogues of phencyclidine: chemical synthesis and pharmacological properties. J Med Chem27:1267-71 (1984) [PubMed]