| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Alpha-mannosidase 2 |
|---|
| Ligand | BDBM50016703 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_32497 (CHEMBL641374) |
|---|
| Ki | 35000±n/a nM |
|---|
| Citation |  Asano, N; Kizu, H; Oseki, K; Tomioka, E; Matsui, K; Okamoto, M; Baba, M N-alkylated nitrogen-in-the-ring sugars: conformational basis of inhibition of glycosidases and HIV-1 replication. J Med Chem38:2349-56 (1995) [PubMed] Asano, N; Kizu, H; Oseki, K; Tomioka, E; Matsui, K; Okamoto, M; Baba, M N-alkylated nitrogen-in-the-ring sugars: conformational basis of inhibition of glycosidases and HIV-1 replication. J Med Chem38:2349-56 (1995) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Alpha-mannosidase 2 |
|---|
| Name: | Alpha-mannosidase 2 |
|---|
| Synonyms: | MA2A1_RAT | Man2a1 | Mana2 | Mannosidase 2 alpha 1 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 131265.36 |
|---|
| Organism: | Rattus norvegicus |
|---|
| Description: | ChEMBL_32391 |
|---|
| Residue: | 1148 |
|---|
| Sequence: | MKLSRQFTVFGSAIFCVVIFSLYLMLDRGHLDYPRGPRQEGSFPQGQLSILQEKIDHLER
LLAENNEIISNIRDSVINLSESVEDGPRGPAGNASQGSAHLHSAQLALQADPKDCLFASQ
SGNQHRDVQMLDVYDLIPFDNPDGGVWKQGFDIKYEADEWDREPLQVFVVPHSHNDPGWL
KTFNDYFRDKTQYIFNNMVLKLKEDSSRKFIWSEISYLAKWWDIIDNPKKEAVKSLLQNG
QLEIVTGGWVMADEATTHYFALIDQLIEGHQWLEKNLGVKPRSGWAIDPFGHSPTMTYLL
KRAGFSHMLIQRVHYSVKKHFSLQKTLEFFWRQNWDLGSTTDILCHMMPFYSYDIPHTCG
PDPKICCQFDFKRLPGGRYGCPWGVPPEAISPGNVQSRAQMLLDQYRKKSKLFRTKVLLA
PLGDDFRFSEYTEWDLQYRNYEQLFSYMNSQPHLKVKIQFGTLSDYFDALEKSVAAEKKG
GQSVFPALSGDFFTYADRDDHYWSGYFTSRPFYKRMDRIMESRLRTAEILYHLALKQAQK
YKINKFLSSPHYTTLTEARRNLGLFQHHDAITGTAKDWVVVDYGTRLFQSLNSLEKIIGD
SAFLLILKDKKLYQSDPSKAFLEMDTKQSSQDSLPKKNIIQLSAQEPRYLVVYNPFEQER
HSVVSVRVNSATVKVLSDLGKAVEVQVSAVWKDMRTTSQAAYEVAFLAHLPPLGLKVYKI
LESQSSSSHLADYFLYNNDGQAESGIFHMKNMVDSGDAITIENSFLTLGFDRSGLMEKVR
RKEDNKQQELKVQFLWYGTTNKRDKSGAYLFLPDGQGQPYVSLRTPFVRVTRGRIYSDVT
CFLEHVTHKVRLYHIQGIEGQSMEVSNIVDIRSVHNREIVMRISSKINNQNRYYTDLNGY
QIQPRRTMAKLPLQANVYPMSTMAYIQDAAHRLTLLSAQSLGASSMASGQIEVFMDRRLM
QDDNRGLGQGVHDNKITANLFRILLEKRNGMNMEEDKKSPVSYPSLLSHMTSAFLNHPFL
PMVLSGQLPSPAIELLSEFRLLQSSLPCDIHLVNLRTIQSKVGKGYSDEAALILHRKVFD
CQLSSRAMGLPCSTTQGKMSIPKLFNNFAVESFIPSSLSLMHSPPDAQNTSEVSLSPMEI
STSRIRLR
|
|
|
|---|
| BDBM50016703 |
|---|
| n/a |
|---|
| Name | BDBM50016703 |
|---|
| Synonyms: | 2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM50031485 | CHEMBL80254 | Imino-D-Arabinitol |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C5H11NO3 |
|---|
| Mol. Mass. | 133.1457 |
|---|
| SMILES | OC[C@H]1NC[C@@H](O)[C@@H]1O |r| |
|---|
| Structure | 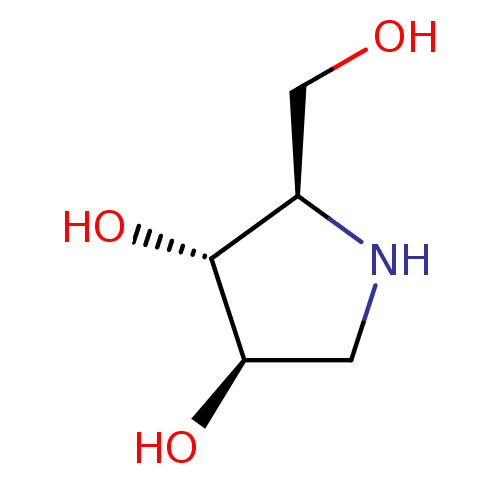
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Asano, N; Kizu, H; Oseki, K; Tomioka, E; Matsui, K; Okamoto, M; Baba, M N-alkylated nitrogen-in-the-ring sugars: conformational basis of inhibition of glycosidases and HIV-1 replication. J Med Chem38:2349-56 (1995) [PubMed]
Asano, N; Kizu, H; Oseki, K; Tomioka, E; Matsui, K; Okamoto, M; Baba, M N-alkylated nitrogen-in-the-ring sugars: conformational basis of inhibition of glycosidases and HIV-1 replication. J Med Chem38:2349-56 (1995) [PubMed]