| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A1 |
|---|
| Ligand | BDBM50051337 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_29152 (CHEMBL639432) |
|---|
| Ki | 1100±n/a nM |
|---|
| Citation |  Karton, Y; Jiang, JL; Ji, XD; Melman, N; Olah, ME; Stiles, GL; Jacobson, KA Synthesis and biological activities of flavonoid derivatives as A3 adenosine receptor antagonists. J Med Chem39:2293-301 (1996) [PubMed] Article Karton, Y; Jiang, JL; Ji, XD; Melman, N; Olah, ME; Stiles, GL; Jacobson, KA Synthesis and biological activities of flavonoid derivatives as A3 adenosine receptor antagonists. J Med Chem39:2293-301 (1996) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A1 |
|---|
| Name: | Adenosine receptor A1 |
|---|
| Synonyms: | AA1R_RAT | ADENOSINE A1 | ADENOSINE A1 high | ADENOSINE A1 low | Adenosine A1 receptor (A1) | Adenosine receptor | Adenosine receptors A1 | Adora1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 36704.13 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | n/a |
|---|
| Residue: | 326 |
|---|
| Sequence: | MPPYISAFQAAYIGIEVLIALVSVPGNVLVIWAVKVNQALRDATFCFIVSLAVADVAVGA
LVIPLAILINIGPQTYFHTCLMVACPVLILTQSSILALLAIAVDRYLRVKIPLRYKTVVT
QRRAAVAIAGCWILSLVVGLTPMFGWNNLSVVEQDWRANGSVGEPVIKCEFEKVISMEYM
VYFNFFVWVLPPLLLMVLIYLEVFYLIRKQLNKKVSASSGDPQKYYGKELKIAKSLALIL
FLFALSWLPLHILNCITLFCPTCQKPSILIYIAIFLTHGNSAMNPIVYAFRIHKFRVTFL
KIWNDHFRCQPKPPIDEDLPEEKAED
|
|
|
|---|
| BDBM50051337 |
|---|
| n/a |
|---|
| Name | BDBM50051337 |
|---|
| Synonyms: | 2-Phenyl-3,5,7-tripropoxy-chromen-4-one | CHEMBL76273 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H28O5 |
|---|
| Mol. Mass. | 396.4761 |
|---|
| SMILES | CCCOc1cc(OCCC)c2c(c1)oc(-c1ccccc1)c(OCCC)c2=O |
|---|
| Structure | 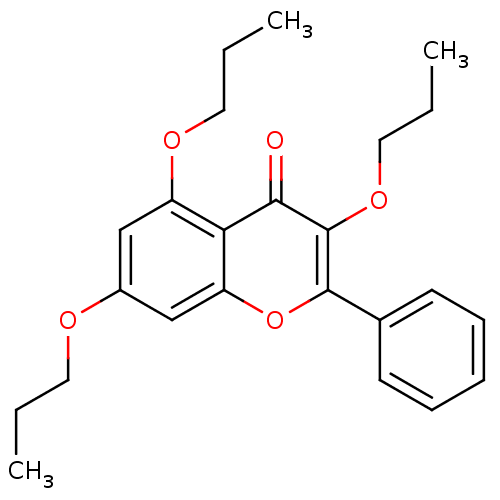
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Karton, Y; Jiang, JL; Ji, XD; Melman, N; Olah, ME; Stiles, GL; Jacobson, KA Synthesis and biological activities of flavonoid derivatives as A3 adenosine receptor antagonists. J Med Chem39:2293-301 (1996) [PubMed] Article
Karton, Y; Jiang, JL; Ji, XD; Melman, N; Olah, ME; Stiles, GL; Jacobson, KA Synthesis and biological activities of flavonoid derivatives as A3 adenosine receptor antagonists. J Med Chem39:2293-301 (1996) [PubMed] Article