

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Acyl-CoA:cholesterol acyltransferase | ||
| Ligand | BDBM50051881 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_28667 (CHEMBL649065) | ||
| IC50 | 25±n/a nM | ||
| Citation |  Astles, PC; Ashton, MJ; Bridge, AW; Harris, NV; Hart, TW; Parrott, DP; Porter, B; Riddell, D; Smith, C; Williams, RJ Acyl-CoA:Cholesterol O-acyltransferase (ACAT) inhibitors. 2. 2-(1,3-Dioxan-2-yl)-4,5-diphenyl-1H-imidazoles as potent inhibitors of ACAT. J Med Chem39:1423-32 (1996) [PubMed] Article Astles, PC; Ashton, MJ; Bridge, AW; Harris, NV; Hart, TW; Parrott, DP; Porter, B; Riddell, D; Smith, C; Williams, RJ Acyl-CoA:Cholesterol O-acyltransferase (ACAT) inhibitors. 2. 2-(1,3-Dioxan-2-yl)-4,5-diphenyl-1H-imidazoles as potent inhibitors of ACAT. J Med Chem39:1423-32 (1996) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Acyl-CoA:cholesterol acyltransferase | |||
| Name: | Acyl-CoA:cholesterol acyltransferase | ||
| Synonyms: | ACAT | ||
| Type: | n/a | ||
| Mol. Mass.: | 35405.31 | ||
| Organism: | Oryctolagus cuniculus | ||
| Description: | n/a | ||
| Residue: | 305 | ||
| Sequence: |
| ||
| BDBM50051881 | |||
| n/a | |||
| Name | BDBM50051881 | ||
| Synonyms: | 1-{4-[2-(4,5-Diphenyl-1H-imidazol-2-yl)-5-methyl-[1,3]dioxan-5-yl]-butyl}-3,5-dimethyl-1H-pyrazole | CHEMBL30860 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C29H34N4O2 | ||
| Mol. Mass. | 470.6059 | ||
| SMILES | Cc1cc(C)n(CCCCC2(C)COC(OC2)c2nc(c([nH]2)-c2ccccc2)-c2ccccc2)n1 |(17.48,-4.71,;16.19,-5.55,;14.76,-4.99,;13.79,-6.2,;12.25,-6.13,;14.64,-7.49,;14.24,-8.98,;12.75,-9.39,;12.35,-10.86,;10.86,-11.26,;10.45,-12.74,;11.77,-13.51,;9.19,-13.16,;7.56,-12.54,;6.79,-13.89,;7.98,-13.47,;9.69,-14.08,;5.45,-13.12,;5.04,-11.63,;3.5,-11.56,;2.96,-12.98,;4.15,-13.96,;1.48,-13.4,;.38,-12.31,;-1.09,-12.7,;-1.49,-14.2,;-.39,-15.28,;1.08,-14.87,;2.66,-10.26,;3.36,-8.88,;2.52,-7.6,;.99,-7.69,;.29,-9.07,;1.13,-10.35,;16.12,-7.08,)| | ||
| Structure | 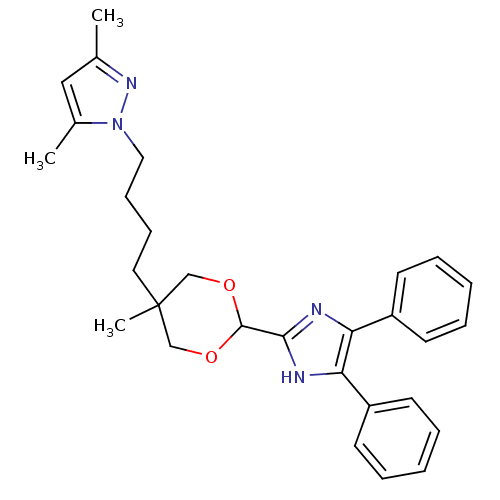
| ||