| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A3 |
|---|
| Ligand | BDBM50053925 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_31845 (CHEMBL641522) |
|---|
| Ki | 3.03±n/a nM |
|---|
| Citation |  Kim, YC; Ji, XD; Jacobson, KA Derivatives of the triazoloquinazoline adenosine antagonist (CGS15943) are selective for the human A3 receptor subtype. J Med Chem39:4142-8 (1996) [PubMed] Article Kim, YC; Ji, XD; Jacobson, KA Derivatives of the triazoloquinazoline adenosine antagonist (CGS15943) are selective for the human A3 receptor subtype. J Med Chem39:4142-8 (1996) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A3 |
|---|
| Name: | Adenosine receptor A3 |
|---|
| Synonyms: | A3 adenosine receptor (hA3) | AA3R_HUMAN | ADORA3 | Adenosine A3 receptor (A3AR) |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 36197.32 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P0DMS8 |
|---|
| Residue: | 318 |
|---|
| Sequence: | MPNNSTALSLANVTYITMEIFIGLCAIVGNVLVICVVKLNPSLQTTTFYFIVSLALADIA
VGVLVMPLAIVVSLGITIHFYSCLFMTCLLLIFTHASIMSLLAIAVDRYLRVKLTVRYKR
VTTHRRIWLALGLCWLVSFLVGLTPMFGWNMKLTSEYHRNVTFLSCQFVSVMRMDYMVYF
SFLTWIFIPLVVMCAIYLDIFYIIRNKLSLNLSNSKETGAFYGREFKTAKSLFLVLFLFA
LSWLPLSIINCIIYFNGEVPQLVLYMGILLSHANSMMNPIVYAYKIKKFKETYLLILKAC
VVCHPSDSLDTSIEKNSE
|
|
|
|---|
| BDBM50053925 |
|---|
| n/a |
|---|
| Name | BDBM50053925 |
|---|
| Synonyms: | CHEMBL317382 | N-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazolin-5-yl)-benzamide | N-(9-chloro-2-(furan-2-yl)-[1,2,4]triazolo[1,5-c]quinazolin-5-yl)benzamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H12ClN5O2 |
|---|
| Mol. Mass. | 389.795 |
|---|
| SMILES | Clc1ccc2nc(NC(=O)c3ccccc3)n3nc(nc3c2c1)-c1ccco1 |
|---|
| Structure | 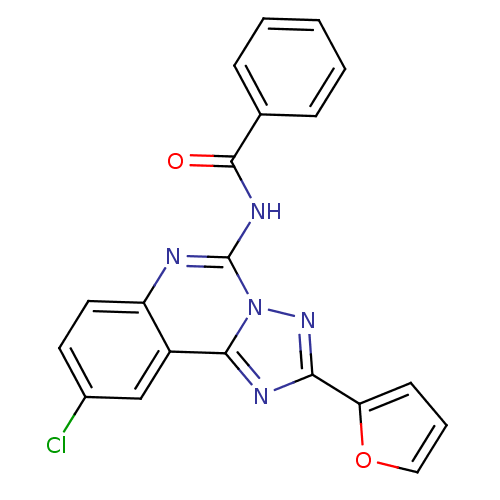
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kim, YC; Ji, XD; Jacobson, KA Derivatives of the triazoloquinazoline adenosine antagonist (CGS15943) are selective for the human A3 receptor subtype. J Med Chem39:4142-8 (1996) [PubMed] Article
Kim, YC; Ji, XD; Jacobson, KA Derivatives of the triazoloquinazoline adenosine antagonist (CGS15943) are selective for the human A3 receptor subtype. J Med Chem39:4142-8 (1996) [PubMed] Article