| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 1A |
|---|
| Ligand | BDBM81993 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1548 (CHEMBL616370) |
|---|
| Ki | 24±n/a nM |
|---|
| Citation |  Heier, RF; Dolak, LA; Duncan, JN; Hyslop, DK; Lipton, MF; Martin, IJ; Mauragis, MA; Piercey, MF; Nichols, NF; Schreur, PJ; Smith, MW; Moon, MW Synthesis and biological activities of (R)-5,6-dihydro-N,N-dimethyl-4H-imidazo[4,5,1-ij]quinolin-5-amine and its metabolites. J Med Chem40:639-46 (1997) [PubMed] Article Heier, RF; Dolak, LA; Duncan, JN; Hyslop, DK; Lipton, MF; Martin, IJ; Mauragis, MA; Piercey, MF; Nichols, NF; Schreur, PJ; Smith, MW; Moon, MW Synthesis and biological activities of (R)-5,6-dihydro-N,N-dimethyl-4H-imidazo[4,5,1-ij]quinolin-5-amine and its metabolites. J Med Chem40:639-46 (1997) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 1A |
|---|
| Name: | 5-hydroxytryptamine receptor 1A |
|---|
| Synonyms: | 5-HT-1A | 5-HT1A | 5-hydroxytryptamine receptor 1A (5-HT-1A) | 5HT1A_HUMAN | ADRB2RL1 | ADRBRL1 | Dopamine D2 receptor and serotonin 1a receptor | G-21 | HTR1A | Serotonin receptor 1A |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 46122.49 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 422 |
|---|
| Sequence: | MDVLSPGQGNNTTSPPAPFETGGNTTGISDVTVSYQVITSLLLGTLIFCAVLGNACVVAA
IALERSLQNVANYLIGSLAVTDLMVSVLVLPMAALYQVLNKWTLGQVTCDLFIALDVLCC
TSSILHLCAIALDRYWAITDPIDYVNKRTPRRAAALISLTWLIGFLISIPPMLGWRTPED
RSDPDACTISKDHGYTIYSTFGAFYIPLLLMLVLYGRIFRAARFRIRKTVKKVEKTGADT
RHGASPAPQPKKSVNGESGSRNWRLGVESKAGGALCANGAVRQGDDGAALEVIEVHRVGN
SKEHLPLPSEAGPTPCAPASFERKNERNAEAKRKMALARERKTVKTLGIIMGTFILCWLP
FFIVALVLPFCESSCHMPTLLGAIINWLGYSNSLLNPVIYAYFNKDFQNAFKKIIKCKFC
RQ
|
|
|
|---|
| BDBM81993 |
|---|
| n/a |
|---|
| Name | BDBM81993 |
|---|
| Synonyms: | BROMOCRIPTINE | Bromocriptine+ (GTP+) | Bromocriptine+ (GTP-) |
|---|
| Type | n/a |
|---|
| Emp. Form. | C32H40BrN5O5 |
|---|
| Mol. Mass. | 654.594 |
|---|
| SMILES | CC(C)C[C@@H]1N2C(=O)[C@](NC(=O)[C@H]3CN(C)[C@@H]4Cc5c(Br)[nH]c6cccc(C4=C3)c56)(O[C@@]2(O)[C@@H]2CCCN2C1=O)C(C)C |r,c:28| |
|---|
| Structure | 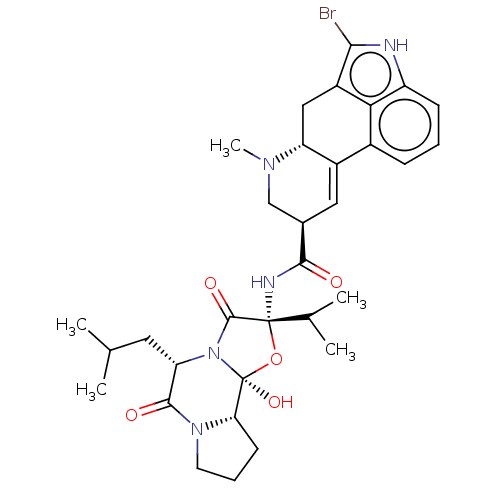
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Heier, RF; Dolak, LA; Duncan, JN; Hyslop, DK; Lipton, MF; Martin, IJ; Mauragis, MA; Piercey, MF; Nichols, NF; Schreur, PJ; Smith, MW; Moon, MW Synthesis and biological activities of (R)-5,6-dihydro-N,N-dimethyl-4H-imidazo[4,5,1-ij]quinolin-5-amine and its metabolites. J Med Chem40:639-46 (1997) [PubMed] Article
Heier, RF; Dolak, LA; Duncan, JN; Hyslop, DK; Lipton, MF; Martin, IJ; Mauragis, MA; Piercey, MF; Nichols, NF; Schreur, PJ; Smith, MW; Moon, MW Synthesis and biological activities of (R)-5,6-dihydro-N,N-dimethyl-4H-imidazo[4,5,1-ij]quinolin-5-amine and its metabolites. J Med Chem40:639-46 (1997) [PubMed] Article