| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM50057819 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_197125 (CHEMBL803726) |
|---|
| EC50 | 1500±n/a nM |
|---|
| Citation |  Adams, DR; Perez, C; Maillard, M; Florent, JC; Evers, M; Hénin, Y; Litvak, S; Litvak, L; Monneret, C; Grierson, DS Preparation and anti-HIV activity of N-3-substituted thymidine nucleoside analogs. J Med Chem40:1550-8 (1997) [PubMed] Article Adams, DR; Perez, C; Maillard, M; Florent, JC; Evers, M; Hénin, Y; Litvak, S; Litvak, L; Monneret, C; Grierson, DS Preparation and anti-HIV activity of N-3-substituted thymidine nucleoside analogs. J Med Chem40:1550-8 (1997) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM50057819 |
|---|
| n/a |
|---|
| Name | BDBM50057819 |
|---|
| Synonyms: | 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-3-(2,2-diethoxy-ethyl)-5-methyl-1H-pyrimidine-2,4-dione | CHEMBL39874 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H25N5O6 |
|---|
| Mol. Mass. | 383.3996 |
|---|
| SMILES | CCOC(Cn1c(=O)c(C)cn(C2CC(N=[N+]=[N-])C(CO)O2)c1=O)OCC |
|---|
| Structure | 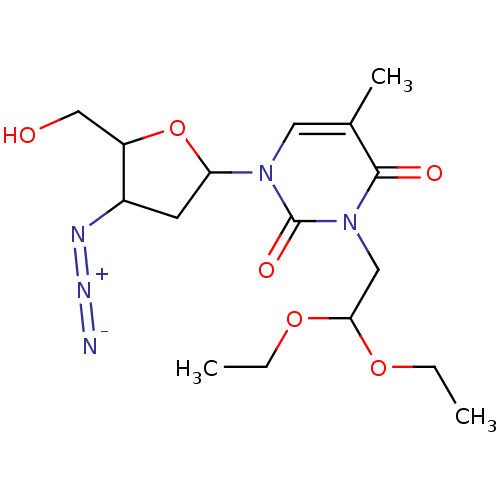
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Adams, DR; Perez, C; Maillard, M; Florent, JC; Evers, M; Hénin, Y; Litvak, S; Litvak, L; Monneret, C; Grierson, DS Preparation and anti-HIV activity of N-3-substituted thymidine nucleoside analogs. J Med Chem40:1550-8 (1997) [PubMed] Article
Adams, DR; Perez, C; Maillard, M; Florent, JC; Evers, M; Hénin, Y; Litvak, S; Litvak, L; Monneret, C; Grierson, DS Preparation and anti-HIV activity of N-3-substituted thymidine nucleoside analogs. J Med Chem40:1550-8 (1997) [PubMed] Article