| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A2a |
|---|
| Ligand | BDBM50053929 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_30017 (CHEMBL641308) |
|---|
| Ki | 52.0±n/a nM |
|---|
| Citation |  Jiang, J; van Rhee, AM; Chang, L; Patchornik, A; Ji, XD; Evans, P; Melman, N; Jacobson, KA Structure-activity relationships of 4-(phenylethynyl)-6-phenyl-1,4-dihydropyridines as highly selective A3 adenosine receptor antagonists. J Med Chem40:2596-608 (1997) [PubMed] Article Jiang, J; van Rhee, AM; Chang, L; Patchornik, A; Ji, XD; Evans, P; Melman, N; Jacobson, KA Structure-activity relationships of 4-(phenylethynyl)-6-phenyl-1,4-dihydropyridines as highly selective A3 adenosine receptor antagonists. J Med Chem40:2596-608 (1997) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A2a |
|---|
| Name: | Adenosine receptor A2a |
|---|
| Synonyms: | AA2AR_RAT | ADENOSINE A2a | Adenosine A2 receptor | Adenosine A2a receptor (A2a) | Adenosine Receptors A2a (A2a) | Adenosine receptor A2a and A3 | Adenosine receptors A2a | Adora2a | Rat striatal adenosine A2a receptor |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 45015.65 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Rat A2A receptors expressed in CHO cells. |
|---|
| Residue: | 410 |
|---|
| Sequence: | MGSSVYITVELAIAVLAILGNVLVCWAVWINSNLQNVTNFFVVSLAAADIAVGVLAIPFA
ITISTGFCAACHGCLFFACFVLVLTQSSIFSLLAIAIDRYIAIRIPLRYNGLVTGVRAKG
IIAICWVLSFAIGLTPMLGWNNCSQKDGNSTKTCGEGRVTCLFEDVVPMNYMVYYNFFAF
VLLPLLLMLAIYLRIFLAARRQLKQMESQPLPGERTRSTLQKEVHAAKSLAIIVGLFALC
WLPLHIINCFTFFCSTCRHAPPWLMYLAIILSHSNSVVNPFIYAYRIREFRQTFRKIIRT
HVLRRQEPFQAGGSSAWALAAHSTEGEQVSLRLNGHPLGVWANGSATHSGRRPNGYTLGL
GGGGSAQGSPRDVELPTQERQEGQEHPGLRGHLVQARVGASSWSSEFAPS
|
|
|
|---|
| BDBM50053929 |
|---|
| n/a |
|---|
| Name | BDBM50053929 |
|---|
| Synonyms: | CHEMBL88147 | N-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazolin-5-yl)-2-phenyl-acetamide | N-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazolin-5-yl)-N-methyl-2-phenyl-acetamide | N-(9-chloro-2-(furan-2-yl)-[1,2,4]triazolo[1,5-c]quinazolin-5-yl)-2-phenylacetamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H14ClN5O2 |
|---|
| Mol. Mass. | 403.821 |
|---|
| SMILES | Clc1ccc2nc(NC(=O)Cc3ccccc3)n3nc(nc3c2c1)-c1ccco1 |
|---|
| Structure | 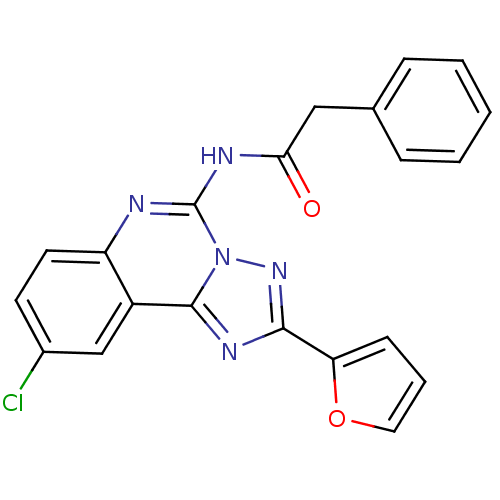
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Jiang, J; van Rhee, AM; Chang, L; Patchornik, A; Ji, XD; Evans, P; Melman, N; Jacobson, KA Structure-activity relationships of 4-(phenylethynyl)-6-phenyl-1,4-dihydropyridines as highly selective A3 adenosine receptor antagonists. J Med Chem40:2596-608 (1997) [PubMed] Article
Jiang, J; van Rhee, AM; Chang, L; Patchornik, A; Ji, XD; Evans, P; Melman, N; Jacobson, KA Structure-activity relationships of 4-(phenylethynyl)-6-phenyl-1,4-dihydropyridines as highly selective A3 adenosine receptor antagonists. J Med Chem40:2596-608 (1997) [PubMed] Article