| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM10245 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_79620 (CHEMBL693549) |
|---|
| EC50 | >20000±n/a nM |
|---|
| Citation |  Roth, T; Morningstar, ML; Boyer, PL; Hughes, SH; Buckheit, RW; Michejda, CJ Synthesis and biological activity of novel nonnucleoside inhibitors of HIV-1 reverse transcriptase. 2-Aryl-substituted benzimidazoles. J Med Chem40:4199-207 (1998) [PubMed] Article Roth, T; Morningstar, ML; Boyer, PL; Hughes, SH; Buckheit, RW; Michejda, CJ Synthesis and biological activity of novel nonnucleoside inhibitors of HIV-1 reverse transcriptase. 2-Aryl-substituted benzimidazoles. J Med Chem40:4199-207 (1998) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM10245 |
|---|
| n/a |
|---|
| Name | BDBM10245 |
|---|
| Synonyms: | 3-(2,6-difluorophenyl)-4-thia-2,7-diazatricyclo[6.4.0.0^{2,6}]dodeca-1(12),6,8,10-tetraene | CHEMBL53554 | NSC 625487 | TBZ | Thiazolobenzimidazole |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H10F2N2S |
|---|
| Mol. Mass. | 288.315 |
|---|
| SMILES | Fc1cccc(F)c1C1SCc2nc3ccccc3n12 |
|---|
| Structure | 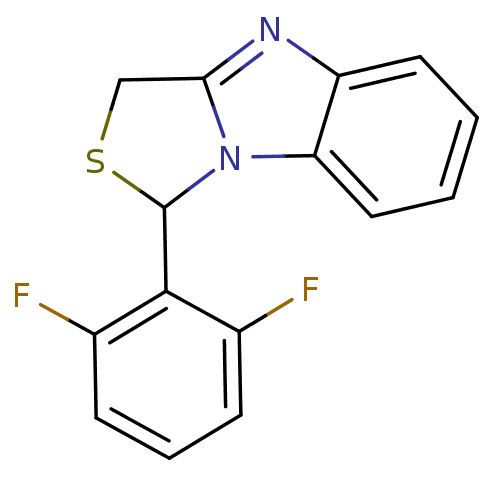
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Roth, T; Morningstar, ML; Boyer, PL; Hughes, SH; Buckheit, RW; Michejda, CJ Synthesis and biological activity of novel nonnucleoside inhibitors of HIV-1 reverse transcriptase. 2-Aryl-substituted benzimidazoles. J Med Chem40:4199-207 (1998) [PubMed] Article
Roth, T; Morningstar, ML; Boyer, PL; Hughes, SH; Buckheit, RW; Michejda, CJ Synthesis and biological activity of novel nonnucleoside inhibitors of HIV-1 reverse transcriptase. 2-Aryl-substituted benzimidazoles. J Med Chem40:4199-207 (1998) [PubMed] Article