| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Kynurenine 3-monooxygenase |
|---|
| Ligand | BDBM50061916 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_91740 (CHEMBL702202) |
|---|
| IC50 | 37±n/a nM |
|---|
| Citation |  Röver, S; Cesura, AM; Huguenin, P; Kettler, R; Szente, A Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J Med Chem40:4378-85 (1998) [PubMed] Article Röver, S; Cesura, AM; Huguenin, P; Kettler, R; Szente, A Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J Med Chem40:4378-85 (1998) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Kynurenine 3-monooxygenase |
|---|
| Name: | Kynurenine 3-monooxygenase |
|---|
| Synonyms: | KMO_RAT | Kmo |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 54371.88 |
|---|
| Organism: | Rattus norvegicus |
|---|
| Description: | ChEMBL_1487468 |
|---|
| Residue: | 478 |
|---|
| Sequence: | MASSDTEGKRVVVIGGGLVGALNACFLAKRNFQVDVYEAREDIRVANFMRGRSINLALSY
RGRQALKAVGLEDQIVSKGVPMKARMIHSLSGKKSAIPYGNKSQYILSISREKLNKDLLT
AVESYPNAKVHFGHKLSKCCPEEGILTMLGPNKVPRDITCDLIVGCDGAYSTVRAHLMKK
PRFDYSQQYIPHGYMELTIPPKNGEYAMEPNCLHIWPRNAFMMIALPNMDKSFTCTLFMS
FEEFEKLPTHSDVLDFFQKNFPDAIPLMGEQALMRDFFLLPAQPMISVKCSPFHLKSRCV
LMGDAAHAIVPFFGQGMNAGFEDCLVFDELMDKFNNDLSVCLPEFSRFRIPDDHAISDLS
MYNYIEMRAHVNSRWFLFQRLLDKFLHALMPSTFIPLYTMVAFTRIRYHEAVLRWHWQKK
VINRGLFVLGSLVAIGSAYILVHHLSPRPLELLRSAWTGTSGHWNRSADISPRVPWSH
|
|
|
|---|
| BDBM50061916 |
|---|
| n/a |
|---|
| Name | BDBM50061916 |
|---|
| Synonyms: | 3,4-Dimethoxy-N-[4-(3-nitro-phenyl)-thiazol-2-yl]-benzenesulfonamide | CHEMBL134915 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H15N3O6S2 |
|---|
| Mol. Mass. | 421.448 |
|---|
| SMILES | COc1ccc(cc1OC)S(=O)(=O)Nc1nc(cs1)-c1cccc(c1)[N+]([O-])=O |
|---|
| Structure | 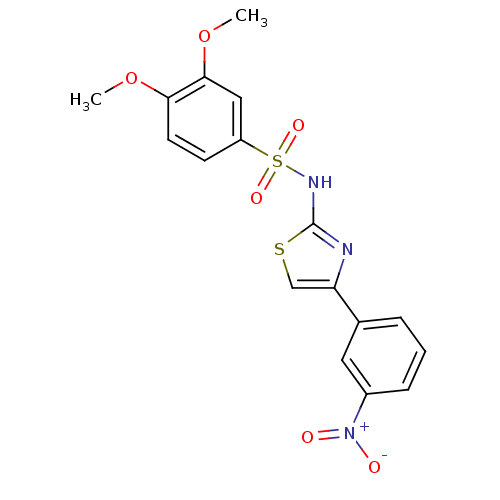
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Röver, S; Cesura, AM; Huguenin, P; Kettler, R; Szente, A Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J Med Chem40:4378-85 (1998) [PubMed] Article
Röver, S; Cesura, AM; Huguenin, P; Kettler, R; Szente, A Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J Med Chem40:4378-85 (1998) [PubMed] Article