| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A3 |
|---|
| Ligand | BDBM50065774 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_31839 (CHEMBL641516) |
|---|
| Ki | 0.468±n/a nM |
|---|
| Citation |  Kim, YC; de Zwart, M; Chang, L; Moro, S; von Frijtag Drabbe Künzel, JK; Melman, N; IJzerman, AP; Jacobson, KA Derivatives of the triazoloquinazoline adenosine antagonist (CGS 15943) having high potency at the human A2B and A3 receptor subtypes. J Med Chem41:2835-45 (1998) [PubMed] Article Kim, YC; de Zwart, M; Chang, L; Moro, S; von Frijtag Drabbe Künzel, JK; Melman, N; IJzerman, AP; Jacobson, KA Derivatives of the triazoloquinazoline adenosine antagonist (CGS 15943) having high potency at the human A2B and A3 receptor subtypes. J Med Chem41:2835-45 (1998) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A3 |
|---|
| Name: | Adenosine receptor A3 |
|---|
| Synonyms: | A3 adenosine receptor (hA3) | AA3R_HUMAN | ADORA3 | Adenosine A3 receptor (A3AR) |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 36197.32 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P0DMS8 |
|---|
| Residue: | 318 |
|---|
| Sequence: | MPNNSTALSLANVTYITMEIFIGLCAIVGNVLVICVVKLNPSLQTTTFYFIVSLALADIA
VGVLVMPLAIVVSLGITIHFYSCLFMTCLLLIFTHASIMSLLAIAVDRYLRVKLTVRYKR
VTTHRRIWLALGLCWLVSFLVGLTPMFGWNMKLTSEYHRNVTFLSCQFVSVMRMDYMVYF
SFLTWIFIPLVVMCAIYLDIFYIIRNKLSLNLSNSKETGAFYGREFKTAKSLFLVLFLFA
LSWLPLSIINCIIYFNGEVPQLVLYMGILLSHANSMMNPIVYAYKIKKFKETYLLILKAC
VVCHPSDSLDTSIEKNSE
|
|
|
|---|
| BDBM50065774 |
|---|
| n/a |
|---|
| Name | BDBM50065774 |
|---|
| Synonyms: | (R)-N-(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinazolin-5-yl)-2-phenyl-propionamide | CHEMBL411692 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H16ClN5O2 |
|---|
| Mol. Mass. | 417.848 |
|---|
| SMILES | C[C@@H](C(=O)Nc1nc2ccc(Cl)cc2c2nc(nn12)-c1ccco1)c1ccccc1 |
|---|
| Structure | 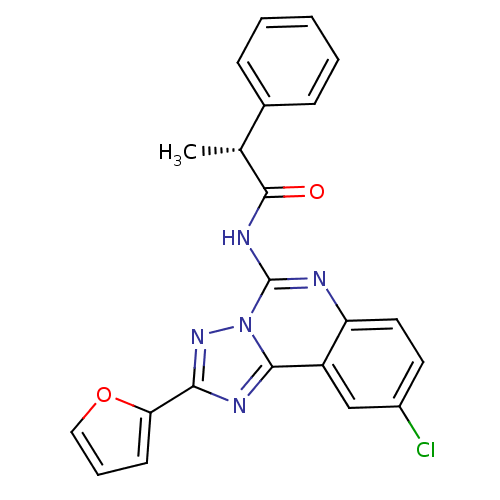
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kim, YC; de Zwart, M; Chang, L; Moro, S; von Frijtag Drabbe Künzel, JK; Melman, N; IJzerman, AP; Jacobson, KA Derivatives of the triazoloquinazoline adenosine antagonist (CGS 15943) having high potency at the human A2B and A3 receptor subtypes. J Med Chem41:2835-45 (1998) [PubMed] Article
Kim, YC; de Zwart, M; Chang, L; Moro, S; von Frijtag Drabbe Künzel, JK; Melman, N; IJzerman, AP; Jacobson, KA Derivatives of the triazoloquinazoline adenosine antagonist (CGS 15943) having high potency at the human A2B and A3 receptor subtypes. J Med Chem41:2835-45 (1998) [PubMed] Article